210218
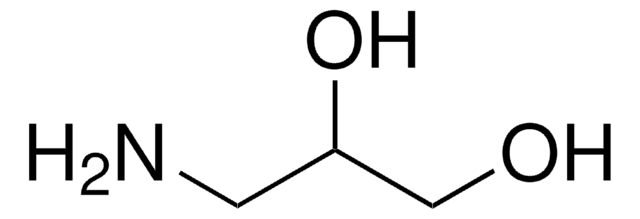
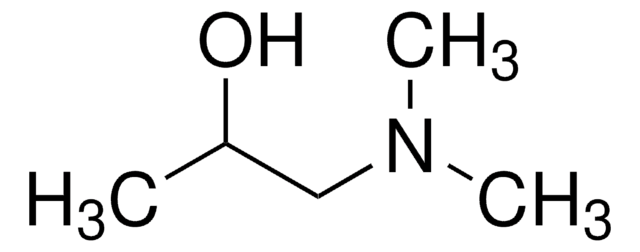
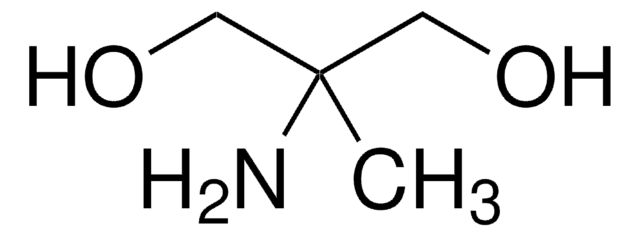
3-(Dimethylamino)-1,2-propanediol
98%
Synonym(s):
(±)-3-(Dimethylamino)-1,2-propanediol, 1,2-Dihydroxy-3-(dimethylamino)propane, Methicol
Sign Into View Organizational & Contract Pricing
All Photos(1)
About This Item
Linear Formula:
(CH3)2NCH2CH(OH)CH2OH
CAS Number:
Molecular Weight:
119.16
EC Number:
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Quality Level
Assay
98%
refractive index
n20/D 1.4609 (lit.)
bp
216-217 °C (lit.)
density
1.004 g/mL at 25 °C (lit.)
functional group
amine
hydroxyl
SMILES string
CN(C)CC(O)CO
InChI
1S/C5H13NO2/c1-6(2)3-5(8)4-7/h5,7-8H,3-4H2,1-2H3
InChI key
QCMHUGYTOGXZIW-UHFFFAOYSA-N
Application
3-(Dimethylamino)-1,2-propanediol was used in the preparation of:
- synthetic cationic lipid, N-[1-(2,3-dioleyloxy)propyl-N,N,N-trimethylammonium chloride (DOTMA), used in DNA-transfection protocol
- indane-derived bis(oxazolines)
Signal Word
Danger
Hazard Statements
Precautionary Statements
Hazard Classifications
Eye Dam. 1 - Skin Corr. 1B
Storage Class Code
8A - Combustible corrosive hazardous materials
WGK
WGK 3
Flash Point(F)
221.0 °F - closed cup
Flash Point(C)
105 °C - closed cup
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
P L Felgner et al.
Proceedings of the National Academy of Sciences of the United States of America, 84(21), 7413-7417 (1987-11-01)
A DNA-transfection protocol has been developed that makes use of a synthetic cationic lipid, N-[1-(2,3-dioleyloxy)propyl]-N,N,N-trimethylammonium chloride (DOTMA). Small unilamellar liposomes containing DOTMA interact spontaneously with DNA to form lipid-DNA complexes with 100% entrapment of the DNA, DOTMA facilitates fusion of
An efficient synthesis of indane-derived bis (oxazoline) and its application to hetero Diels-Alder reactions on polymer support.
Kurosu M, et al.
Tetrahedron Letters, 45(1), 145-148 (2004)
Shuo Fang et al.
International journal of pharmaceutics, 493(1-2), 460-465 (2015-08-11)
A novel dual drug-tailed betaine conjugate amphiphile has been firstly synthesized in which the polar headgroup is derived from glycine betaine and the hydrophobic tails are chlorambucil molecules. The newly prepared conjugate undergoes self-assembly to form stable liposome-like nanocapsules as
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service