All Photos(2)
About This Item
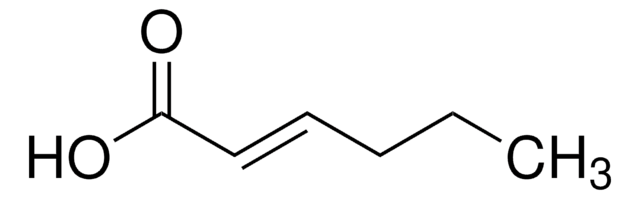
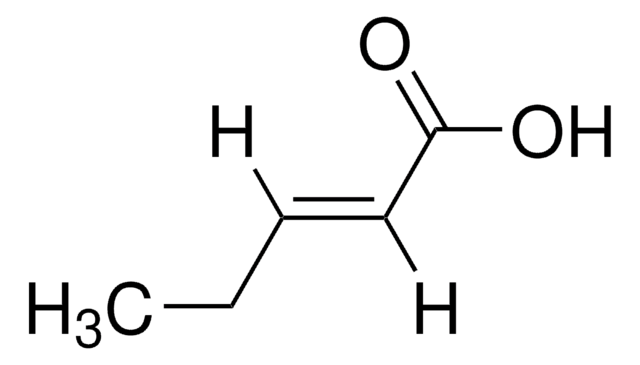
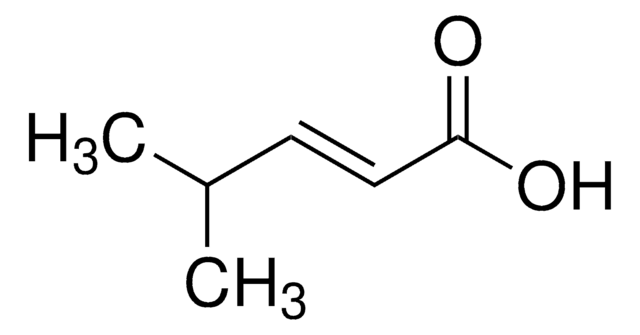
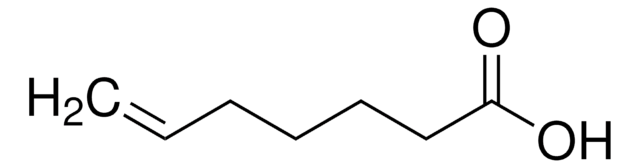
Linear Formula:
CH3CH2CH2CH=CHCO2H
CAS Number:
Molecular Weight:
114.14
Beilstein:
1720443
EC Number:
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
vapor density
>1 (vs air)
Quality Level
Assay
99%
form
solid
refractive index
n20/D 1.438 (lit.)
bp
217 °C (lit.)
mp
33-35 °C (lit.)
density
0.965 g/mL at 25 °C (lit.)
functional group
carboxylic acid
storage temp.
2-8°C
SMILES string
CCC\C=C\C(O)=O
InChI
1S/C6H10O2/c1-2-3-4-5-6(7)8/h4-5H,2-3H2,1H3,(H,7,8)/b5-4+
InChI key
NIONDZDPPYHYKY-SNAWJCMRSA-N
Looking for similar products? Visit Product Comparison Guide
Signal Word
Danger
Hazard Statements
Precautionary Statements
Hazard Classifications
Skin Corr. 1B
Storage Class Code
8A - Combustible corrosive hazardous materials
WGK
WGK 3
Flash Point(F)
235.4 °F - closed cup
Flash Point(C)
113 °C - closed cup
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Saloni Pasta et al.
Chemistry & biology, 14(12), 1377-1385 (2007-12-22)
Expression, characterization, and mutagenesis of a series of N-terminal fragments of an animal fatty acid synthase, containing the beta-ketoacyl synthase, acyl transferase, and dehydratase domains, demonstrate that the dehydratase domain consists of two pseudosubunits, derived from contiguous regions of the
Rhodium-catalyzed asymmetric 1, 4-addition of arylboron reagents to α,β-unsaturated esters.
Takaya Y, et al.
Tetrahedron Asymmetry, 10(20), 4047-4056 (1999)
Biooxidation of primary alcohols to aldehydes through hydrogen transfer employing janibacter terrae.
Orbegozo T, et al.
European Journal of Organic Chemistry, 2010(18), 3445-3448 (2010)
Fangfang Zeng et al.
Insect biochemistry and molecular biology, 113, 103213-103213 (2019-08-24)
Mosquitoes rely heavily on the olfactory system to find a host for a bloodmeal, plants for a source of energy and suitable sites for oviposition. Here, we examined a cluster of eight odorant receptors (ORs), which includes one OR, CquiOR1
Pingxi Xu et al.
Proceedings of the National Academy of Sciences of the United States of America, 111(46), 16592-16597 (2014-10-29)
Insect repellents are important prophylactic tools for travelers and populations living in endemic areas of malaria, dengue, encephalitis, and other vector-borne diseases. DEET (N,N-diethyl-3-methylbenzamide) is a 6-decade-old synthetic repellent, which is still considered the gold standard of mosquito repellents. Mosquitoes
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service