185493
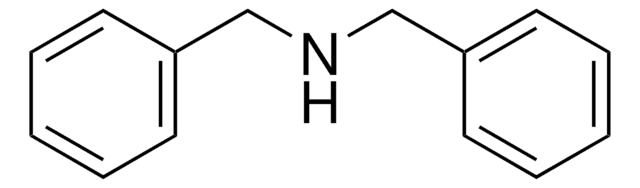
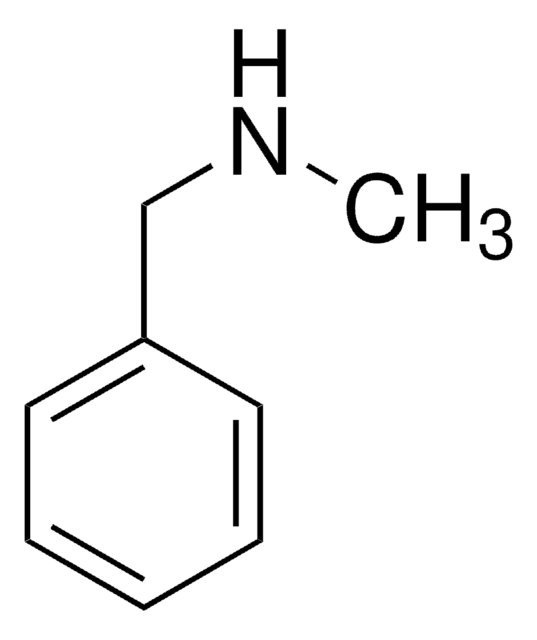
N-Benzylaniline
≥99%
Synonym(s):
N-Phenylbenzylamine
Sign Into View Organizational & Contract Pricing
All Photos(2)
About This Item
Linear Formula:
C6H5CH2NHC6H5
CAS Number:
Molecular Weight:
183.25
EC Number:
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Quality Level
Assay
≥99%
form
solid
bp
306-307 °C (lit.)
mp
35-38 °C (lit.)
solubility
alcohol: soluble
chloroform: soluble
diethyl ether: soluble
water: insoluble
density
1.061 g/mL at 25 °C (lit.)
functional group
amine
phenyl
SMILES string
C(Nc1ccccc1)c2ccccc2
InChI
1S/C13H13N/c1-3-7-12(8-4-1)11-14-13-9-5-2-6-10-13/h1-10,14H,11H2
InChI key
GTWJETSWSUWSEJ-UHFFFAOYSA-N
Looking for similar products? Visit Product Comparison Guide
Related Categories
General description
The electropolymerisation of N-benzylaniline at transparent Indium Tin Oxide glass electrodes has been investigated by UV-visible spectroelectrochemistry. N-Benzylaniline on electrochemical oxidation in aqueous sulfuric acid solution produces an adherent conducting polymer film at the platinum electrode.
Application
N-Benzylaniline was used in the separation of tervalent gallium, indium and thallium by solvent extraction method.
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Flash Point(F)
235.4 °F - closed cup
Flash Point(C)
113 °C - closed cup
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
M M Khosla et al.
Talanta, 21(6), 411-415 (1974-06-01)
A simple and rapid method is proposed for the separation of tervalent gallium, indium and thallium by solvent extraction with N-benzylaniline in chloroform from different concentrations of hydrochloric acid. Thallium and gallium are extracted from 1M and 7.0-7.5M hydrochloric acid
A UV-visible spectroelectrochemical study of the electropolymerisation of N-benzylaniline.
Malinauskas A and Holze R.
Journal of Solid State Electrochemistry, 3(7-8), 429-436 (1999)
Electrochemical polymerization and characterization of N-benzylaniline.
Dong S and Li Z.
Synthetic Metals, 33(1), 93-98 (1989)
Synthesis and structure-activity relationships of a class of sodium iodide symporter function inhibitors.
Fanny Waltz et al.
ChemMedChem, 6(10), 1775-1777 (2011-07-14)
M Ulgen et al.
Xenobiotica; the fate of foreign compounds in biological systems, 24(8), 735-748 (1994-08-01)
1. The in vitro hepatic microsomal metabolism of certain substituted N-benzylanilines was studied in the male hamster to establish the mechanism(s) and process(es) involved in the formation of the corresponding amides. 2. N-Benzyl-2,4,6-trihalogeno, N-benzyl-4-cyano- and N-benzyl-4-nitroanilines were only metabolized by
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service