18509
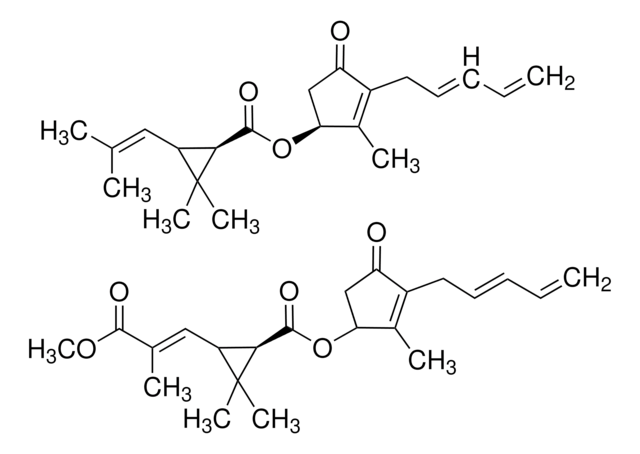
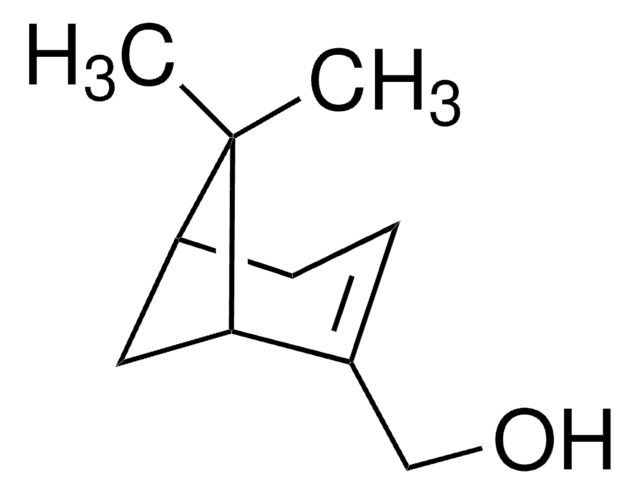
(+)-trans-Chrysanthemic acid
≥97.0% (GC)
Synonym(s):
(1R,3R)-trans-2,2-Dimethyl-3-(2-methyl-1-propenyl)cyclopropane-1-carboxylic acid
Sign Into View Organizational & Contract Pricing
All Photos(1)
About This Item
Empirical Formula (Hill Notation):
C10H16O2
CAS Number:
Molecular Weight:
168.23
Beilstein:
4904351
EC Number:
MDL number:
UNSPSC Code:
12352002
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Quality Level
Assay
≥97.0% (GC)
optical activity
[α]/D +14±1°, c = 2% in ethanol
refractive index
n20/D 1.477
functional group
carboxylic acid
SMILES string
C\C(C)=C/[C@@H]1[C@@H](C(O)=O)C1(C)C
InChI
1S/C10H16O2/c1-6(2)5-7-8(9(11)12)10(7,3)4/h5,7-8H,1-4H3,(H,11,12)/t7-,8+/m1/s1
InChI key
XLOPRKKSAJMMEW-SFYZADRCSA-N
General description
(+)-trans-Chrysanthemic acid is the acidic component of synthetic pyrethroid insecticides. It can be prepared from (+)-Δ3-carene.
Application
(+)-trans-Chrysanthemic acid may be used in the preparation of (+)-trans-pyrethric acid, a building block for rethrin II.
Packaging
Bottomless glass bottle. Contents are inside inserted fused cone.
Signal Word
Warning
Hazard Statements
Precautionary Statements
Hazard Classifications
Eye Irrit. 2 - Skin Irrit. 2
Storage Class Code
10 - Combustible liquids
WGK
WGK 3
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Wolfgang Bicker et al.
Journal of chromatography. A, 1035(1), 37-46 (2004-05-01)
This study reports on the direct HPLC stereoisomer separation of selected pyrethroic acids employing commercial cinchona alkaloid derived chiral stationary phases (CSPs). cis/trans-Chrysanthemic acid (cis/trans-CA), cis/trans-chrysanthemum dicarboxylic acid (cis/trans-CDCA), cis/trans-permethrinic acid (cis/trans-PA), and fenvaleric acid (FA) were resolved into the
[Evaluation of the toxicity of chrysanthemic acid and its derivatives].
A I Gurova et al.
Gigiena i sanitariia, (1)(1), 16-18 (1986-01-01)
Studies on Chrysanthemic Acid: Part VIII. Synthesis of Pyrethric Acids Part IX. Alternate Preparation of (+)-trans-pyrethric Acid and Methyl (+)-trans-2, 2-Dimethyl-3-(2'-carboxy-1' propenyl)-cyclopropanecarboxylate.
Matsui M, et al.
Agricultural and Biological Chemistry, 27(5), 373-380 (1963)
M Dondi et al.
Journal of chromatography. A, 859(2), 133-142 (1999-11-26)
The direct enantioseparation of chrysanthemic acid [2,2-dimethyl-3-(2-methylpropenyl)-cyclopropanecarboxylic acid] and its halogen-substituted analogues was systematically studied by HPLC using a terguride-based chiral stationary phase in combination with a UV diode array and chiroptical detectors. Isomers with (1R) configuration always eluted before
M Nishizawa et al.
Applied and environmental microbiology, 61(9), 3208-3215 (1995-09-01)
The gene coding for a novel esterase which stereoselectively hydrolyzes the (+)-trans (1R,3R) stereoisomer of ethyl chrysanthemate was cloned from Arthrobacter globiformis SC-6-98-28 and overexpressed in Escherichia coli. The cellular content of the active enzyme reached 33% of the total
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service