130737
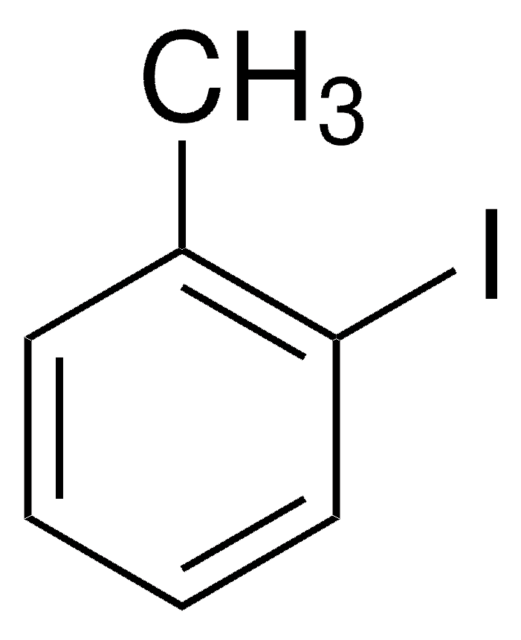
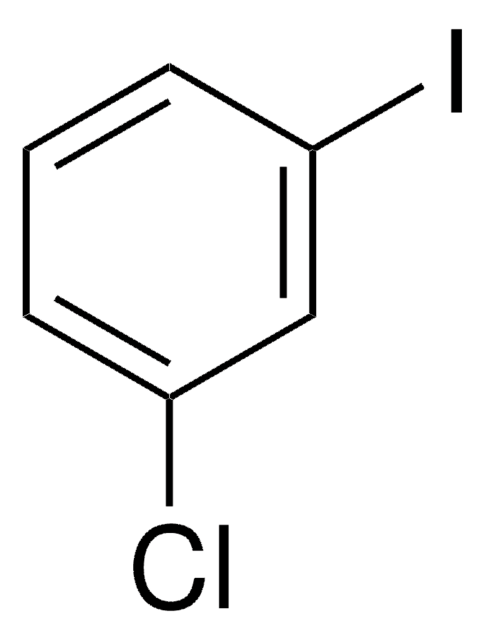
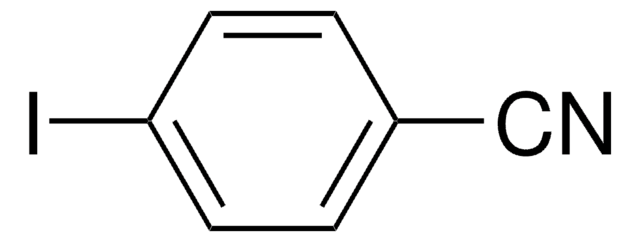
3-Iodotoluene
99%
Synonym(s):
1-Iodo-3-methylbenzene
Sign Into View Organizational & Contract Pricing
All Photos(1)
About This Item
Linear Formula:
CH3C6H4I
CAS Number:
Molecular Weight:
218.03
Beilstein:
1903634
EC Number:
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Quality Level
Assay
99%
form
liquid
refractive index
n20/D 1.604 (lit.)
bp
80-82 °C/10 mmHg (lit.)
density
1.698 g/mL at 25 °C (lit.)
functional group
iodo
SMILES string
Cc1cccc(I)c1
InChI
1S/C7H7I/c1-6-3-2-4-7(8)5-6/h2-5H,1H3
InChI key
VLCPISYURGTGLP-UHFFFAOYSA-N
Looking for similar products? Visit Product Comparison Guide
Application
3-Iodotoluene (1-Iodo-3-methylbenzene) was used in the preparartion of indene derivatives.
Signal Word
Warning
Hazard Statements
Precautionary Statements
Hazard Classifications
Eye Irrit. 2 - Skin Irrit. 2 - STOT SE 3
Target Organs
Respiratory system
Storage Class Code
10 - Combustible liquids
WGK
WGK 2
Flash Point(F)
181.4 °F - closed cup
Flash Point(C)
83 °C - closed cup
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Ya-Jun Liu et al.
The Journal of chemical physics, 132(1), 014306-014306 (2010-01-19)
The multistate second order multiconfigurational perturbation theory in conjunction with spin-orbit interaction through complete active space state interaction (MS-CASPT2/CASSI-SO) was employed to calculate the potential energy curves for the ground and low-lying excited states of o-, m-, and p-iodotoluene along
Zheng-Hui Guan et al.
Organic & biomolecular chemistry, 6(6), 1040-1045 (2008-03-11)
The palladium-catalyzed carboannulation and arylation reaction of propargylic carbonates with in situ generated organozinc compounds produced an important new class of indene derivatives. The reaction proceeded under mild conditions, and indene products were isolated in good to excellent yields.
[Information on basics of MAC for m-toluate iodide in the air of work area].
V I Sokolov et al.
Gigiena truda i professional'nye zabolevaniia, (9-10)(9-10), 44-45 (1992-01-01)
Mizoroki-heck-type reaction mediated by potassium tert-butoxide.
Eiji Shirakawa et al.
Angewandte Chemie (International ed. in English), 50(20), 4671-4674 (2011-04-08)
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service