130036
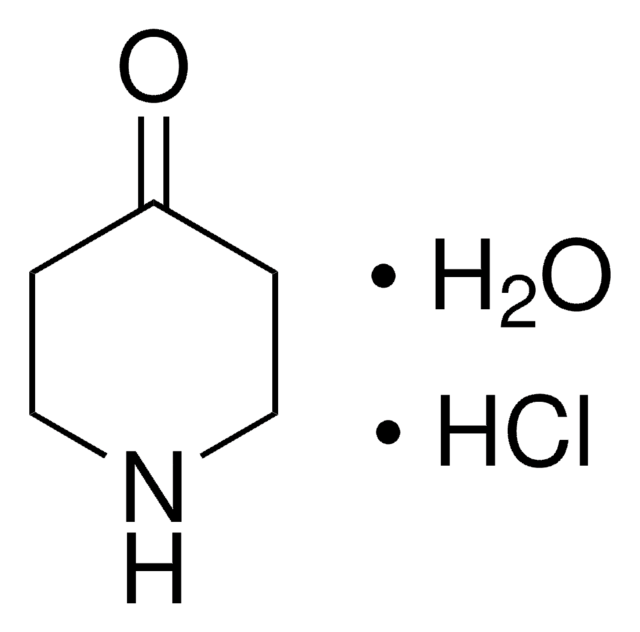
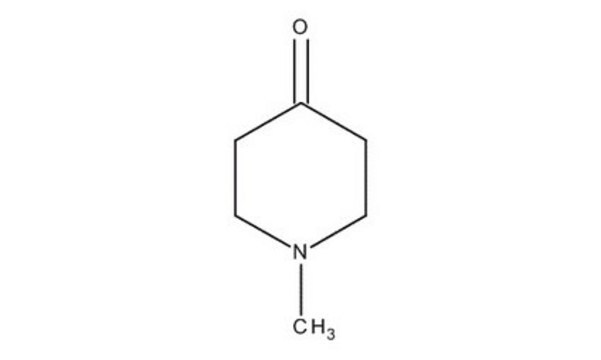
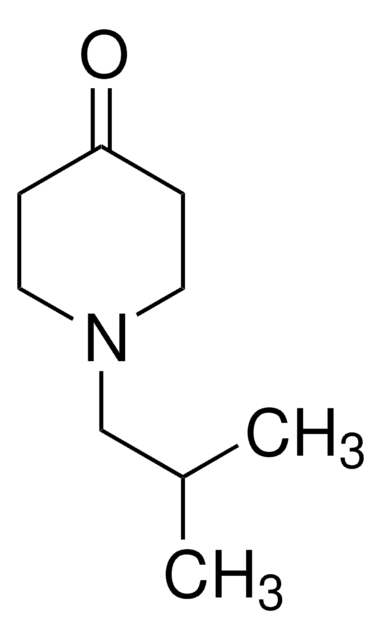
N-Methyl-4-piperidone
97%
Synonym(s):
1-Methyl-4-piperidinone
Sign Into View Organizational & Contract Pricing
All Photos(1)
About This Item
Empirical Formula (Hill Notation):
C6H11NO
CAS Number:
Molecular Weight:
113.16
Beilstein:
106924
EC Number:
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
reg. compliance
suitable for FDA C-010.02
Quality Level
Assay
97%
form
liquid
density
0.92 g/mL at 25 °C (lit.)
functional group
ketone
storage temp.
2-8°C
SMILES string
CN1CCC(=O)CC1
InChI
1S/C6H11NO/c1-7-4-2-6(8)3-5-7/h2-5H2,1H3
InChI key
HUUPVABNAQUEJW-UHFFFAOYSA-N
Application
N-Methyl-4-piperidone can be used as a reactant to prepare:
- Spiropiperidine rings by reacting with malononitrile and electrophiles or Michael acceptors.
- (3E,5E)-1-Methyl-3,5-bis(phenylmethylene)-4-piperidinone by reacting with benzaldehyde via Michael addition, followed by intramolecular O-cyclization/elimination sequential reactions.
- N,N′-Dimethylbispidinone by utilizing a double Mannich condensation method.
Signal Word
Warning
Hazard Statements
Hazard Classifications
Flam. Liq. 3
Storage Class Code
3 - Flammable liquids
WGK
WGK 3
Flash Point(F)
136.4 °F - closed cup
Flash Point(C)
58 °C - closed cup
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Bin-Rong Yao et al.
European journal of medicinal chemistry, 167, 187-199 (2019-02-17)
To get new anti-hepatoma agents with anti-inflammatory activity and hypotoxicity, a series of dissymmetric pyridyl-substituted 3,5-bis(arylidene)-4-piperidones (BAPs, 25-82) were designed and synthesized. Many of them exhibited potential anti-hepatoma properties against human hepatocellular carcinoma cell lines (HepG2, QGY-7703, SMMC-7721) and hypotoxicity
Analogs of sparteine. I. A reexamination of the reaction of n-methyl-4-piperidone with formaldehyde and methylamine. A revised synthesis of n,n'-dimethylbispidinone.
E E Smissman et al.
The Journal of organic chemistry, 40(2), 251-252 (1975-01-24)
Chang-Ming Su et al.
Journal of enzyme inhibition and medicinal chemistry, 34(1), 1287-1297 (2019-07-11)
Inhibition of NF-κB signalling has been demonstrated as a therapeutic option in treating inflammatory diseases and cancers. Herein, we synthesized novel dissymmetric 3,5-bis(arylidene)-4-piperidones (BAPs, 83-102) and characterized fully. MTT and ELISA assay were performed to screen the anti-hepatoma and anti-inflammation
Ning Li et al.
European journal of medicinal chemistry, 155, 531-544 (2018-06-18)
Ten novel symmetric 3,5-bis(arylidene)-4-piperidone derivatives (BAPs, 1-10) and fourteen dissymmetric BAPs (11-24) were synthesized and evaluated the cytotoxicity. All of the compounds have been screened for their anti-inflammatory activity characterized by evaluating their inhibitory effects on LPS-induced IL-6, TNF-α secretion.
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service