All Photos(1)
About This Item
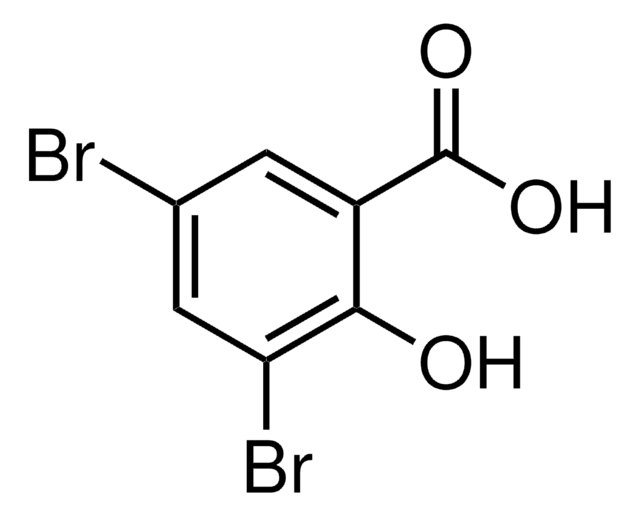
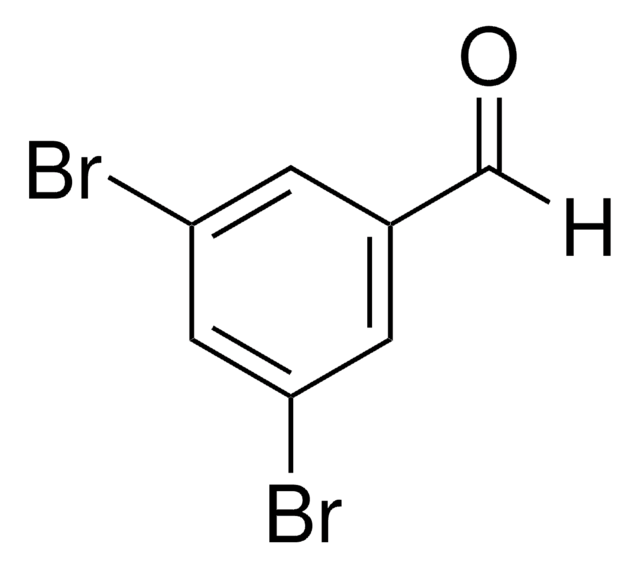
Linear Formula:
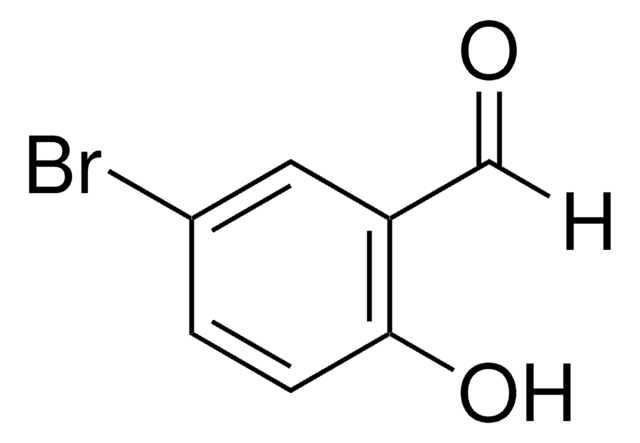
Br2C6H2-2-(OH)CHO
CAS Number:
Molecular Weight:
279.91
EC Number:
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Quality Level
Assay
98%
form
solid
mp
82-83.5 °C (lit.)
solubility
methanol: soluble 25 mg/mL, clear, yellow to brown
functional group
aldehyde
bromo
SMILES string
Oc1c(Br)cc(Br)cc1C=O
InChI
1S/C7H4Br2O2/c8-5-1-4(3-10)7(11)6(9)2-5/h1-3,11H
InChI key
JHZOXYGFQMROFJ-UHFFFAOYSA-N
General description
3,5-Dibromosalicylaldehyde reacts with alkyl cyanoacetates in the presence of ammonium acetate to yield 4H- chromenes.
Application
3,5-Dibromosalicylaldehyde was used in the synthesis of Schiff base, 15N-Labeled N-(3,5-dibromosalicylidene)-methylamine. It was used as reactant for synthesis of Schiff base ligands which forms mononuclear complexes with copper(II), nickel(II), zinc(II) and cobalt(II).
Signal Word
Warning
Hazard Statements
Precautionary Statements
Hazard Classifications
Aquatic Acute 1 - Eye Irrit. 2 - Skin Irrit. 2 - STOT SE 3
Target Organs
Respiratory system
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Shasad Sharif et al.
Journal of the American Chemical Society, 128(10), 3375-3387 (2006-03-09)
The tautomeric equilibrium in a Schiff base, N-(3,5-dibromosalicylidene)-methylamine 1, a model for the hydrogen bonded structure of the cofactor pyridoxal-5'-phosphate PLP which is located in the active site of the enzyme, was measured by means of 1H and 15N NMR
Z Rozwadowski
Magnetic resonance in chemistry : MRC, 44(9), 881-886 (2006-06-03)
Deuterium isotope effects on 13C chemical shift of tetrabutylammonium salts of Schiff bases, derivatives of amino acids (glycine, L-alanine, L-phenylalanine, L-valine, L-leucine, L-isoleucine and L-methionine) and various ortho-hydroxyaldehydes in CDCl3 have been measured. The results have shown that the tetrabutylammonium
Haibo Ni et al.
Experimental neurology, 304, 102-113 (2018-03-09)
Receptor for activated protein kinase C 1 (RACK1) is a multifaceted scaffolding protein known to be involved in the regulation of signaling events required for neuronal protection. In the present study, we investigated the role of RACK1 in secondary brain
[Colorimetric determination of 4-chloro-2-(o-chlorobenzoyl)-N-methyl-N alpha-glycylglycinanilide with 3,5-dibromsalicyladehyde (author's transl)].
R Ikenishi et al.
Yakugaku zasshi : Journal of the Pharmaceutical Society of Japan, 101(6), 532-537 (1981-06-01)
Qing Liu et al.
Acta chimica Slovenica, 63(2), 279-286 (2016-06-23)
Two new cis-dioxomolybdenum(VI) complexes with general formula [MoO2L(MeOH)], where L = L1 = N'-(3,5-dibromo-2-hydroxybenzylidene)-4-trifluoromethylbenzohydrazide for complex 1, and L = L2 = N'-(3-bromo-5-chloro-2-hydroxybenzylidene)-4-trifluoromethylbenzohydrazide for complex 2, have been synthesized and fully characterized on the basis of elemental analysis, FT-IR, molar
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service