102601
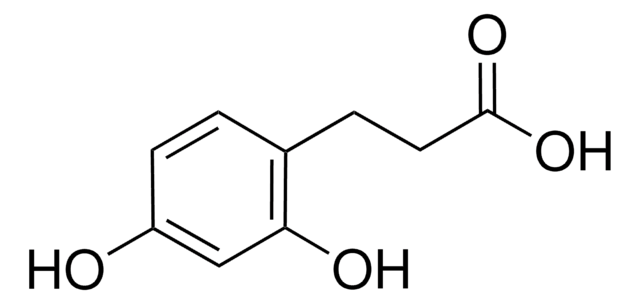
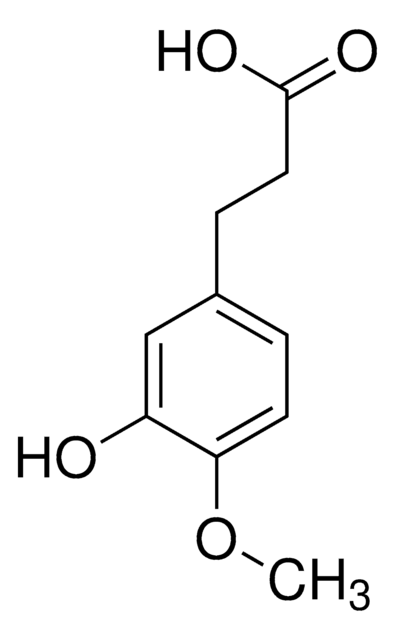
3,4-Dihydroxyhydrocinnamic acid
98%
Synonym(s):
3-(3,4-Dihydroxyphenyl)propionic acid, Hydrocaffeic acid
Sign Into View Organizational & Contract Pricing
All Photos(1)
About This Item
Linear Formula:
(HO)2C6H3CH2CH2CO2H
CAS Number:
Molecular Weight:
182.17
Beilstein:
2213449
EC Number:
MDL number:
UNSPSC Code:
12162002
PubChem Substance ID:
NACRES:
NA.23
Recommended Products
Quality Level
Assay
98%
mp
136-139 °C (lit.)
SMILES string
OC(=O)CCc1ccc(O)c(O)c1
InChI
1S/C9H10O4/c10-7-3-1-6(5-8(7)11)2-4-9(12)13/h1,3,5,10-11H,2,4H2,(H,12,13)
InChI key
DZAUWHJDUNRCTF-UHFFFAOYSA-N
Looking for similar products? Visit Product Comparison Guide
Related Categories
General description
3,4-Dihydroxyhydrocinnamic acid (DHCA) belongs to the class of monomers known as hydroxycinnamic acids. DHCA, also known as hydrocaffeic acid, can be polymerized to form various caffeic acid-based polymers. These polymers can be used in the development of coatings, films, and packaging materials due to their antioxidant and antimicrobial properties. caffeic acid-based polymers have also shown potential applications in drug delivery systems, tissue engineering, and encapsulation of bioactive compounds. Additionally, these polymers can be incorporated into cosmetic and personal care products due to their antioxidant and anti-inflammatory properties.
Application
3,4-Dihydroxyhydrocinnamic acid(DHCA) can be used as a monomer to prepare muscle-mimetic, bioadhesive polymers. The strong adhesive action of the polymers is due to interactions of catechol groups present at the end of the polymer terminal chain and substrate surface.
It can also be used for thesurface functionalization of nanoparticles. The functionalization with DHCA notonly improves the water solubility of nanoparticles but also provides a platformfor further modification due to the presence of surface carboxyl groups.
It can also be used for thesurface functionalization of nanoparticles. The functionalization with DHCA notonly improves the water solubility of nanoparticles but also provides a platformfor further modification due to the presence of surface carboxyl groups.
Signal Word
Warning
Hazard Statements
Precautionary Statements
Hazard Classifications
Eye Irrit. 2 - Skin Irrit. 2 - STOT SE 3
Target Organs
Respiratory system
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Kebba Sabally et al.
Journal of biotechnology, 127(1), 167-176 (2006-08-15)
Lipase-catalyzed transesterification reaction of dihydrocaffeic acid (DHCA) with flaxseed oil in organic solvent media was investigated. Using equal molar concentration of DHCA and flaxseed oil, only phenolic monoacylglycerols were obtained with a transesterification yield (TY) of 18.9%. A 1:4 DHCA
Reina Aoki et al.
The Journal of neuroscience : the official journal of the Society for Neuroscience, 31(46), 16603-16610 (2011-11-18)
The ability to detect harmful chemicals rapidly is essential for the survival of all animals. In Caenorhabditis elegans (C. elegans), repellents trigger an avoidance response, causing animals to move away from repellents. Dihydrocaffeic acid (DHCA) is a water-soluble repellent and
Charlotte S Hunneche et al.
Journal of agricultural and food chemistry, 56(19), 9258-9268 (2008-09-12)
A combinatorial chemistry approach was employed for the design and systematic synthesis of antioxidant-emulsifier bioconjugates to improve antioxidant activity in the interface between oil and water. A combinatorial library of 12 bioconjugates was synthesized from the phenolic antioxidants Trolox (a
Lee E Hunt et al.
Biomolecules, 11(1) (2021-01-06)
There is ongoing interest in exploiting the antioxidant activity and other medicinal properties of natural monophenolic/polyphenolic compounds, but their generally low aqueous solubility limits their applications. Numerous studies have been undertaken to solubilize such compounds via supramolecular derivatization with co-crystal
María Monagas et al.
The British journal of nutrition, 102(2), 201-206 (2009-07-10)
Oligomers and polymers of flavan-3-ols (proanthocyanidins) are very abundant in the Mediterranean diet, but are poorly absorbed. However, when these polyphenols reach the colon, they are metabolised by the intestinal microbiota into various phenolic acids, including phenylpropionic, phenylacetic and benzoic
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service