T5772
Thrombin from rat plasma
lyophilized powder, ≥1,000 units/mg protein (biuret)
Sign Into View Organizational & Contract Pricing
All Photos(1)
About This Item
Recommended Products
General description
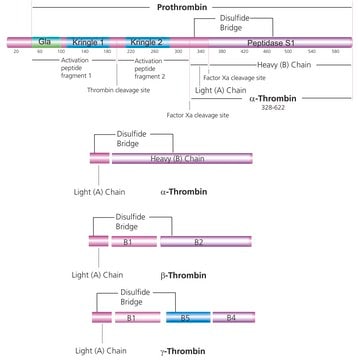
Thrombin is the final coagulation protease in regard to hemostasis, promoting both procoagulant and anticoagulant effects.
Application
Thrombin is used for site specific cleavage of recombinant fusion proteins containing an accessible thrombin recognition site for removal of affinity tags. Thrombin has been used in a study to investigate activation of equine platelet-rich plasma.
Thrombin is used for site specific cleavage of recombinant fusion proteins containing an accessible thrombin recognition site for removal of affinity tags.
Biochem/physiol Actions
Serine protease that selectively cleaves Arg-Gly bonds in fibrinogen to form fibrin and fibrinopeptides A and B.
Unit Definition
Activity is expressed in NIH units obtained by direct comparison to a NIH Thrombin Reference Standard, Lot K.
Physical form
Lyophilized from saline sodium citrate buffer, pH 6.5
Analysis Note
The NIH assay procedure uses 0.2 ml diluted plasma (1:1 with saline) as a substrate and 0.1 ml of thrombin sample (stabilized in a 1% buffered albumin solution) based on a modification of the method of Biggs. Only clotting times in the range of 15-25 seconds are used for determining thrombin concentrations.
Other Notes
View more information on thrombin at www.sigma-aldrich.com/enzymeexplorer.
inhibitor
Product No.
Description
Pricing
Signal Word
Danger
Hazard Statements
Precautionary Statements
Hazard Classifications
Eye Irrit. 2 - Resp. Sens. 1 - Skin Irrit. 2 - STOT SE 3
Target Organs
Respiratory system
Storage Class Code
11 - Combustible Solids
WGK
WGK 2
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Certificates of Analysis (COA)
Search for Certificates of Analysis (COA) by entering the products Lot/Batch Number. Lot and Batch Numbers can be found on a product’s label following the words ‘Lot’ or ‘Batch’.
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Jamie A Textor et al.
Veterinary surgery : VS, 41(7), 784-794 (2012-06-30)
To investigate and compare clinically relevant Platelet-rich plasma (PRP) activation methods. Experimental. PRP was prepared from 6 equine subjects. Activation of the PRP was performed by 4 methods (autologous thrombin, bovine thrombin, calcium chloride (CaCl(2) ), or freeze-thaw). The resultant
Xiaojun Chen et al.
Cell death discovery, 8(1), 189-189 (2022-04-12)
Spinal cord injury (SCI) will result in the significant elevation of thrombin production at lesion site via either breakage of blood-spinal cord barrier or upregulated expression within nerve cells. Thrombin-induced activation of the protease activated receptors (PARs) evokes various pathological
Response.
Sarah T Garber et al.
Journal of neurosurgery, 118(2), 485-485 (2013-03-16)
Dilyara Cheranova et al.
Journal of visualized experiments : JoVE, (72)(72), doi:10-doi:10 (2013-02-22)
The characterization of gene expression in cells via measurement of mRNA levels is a useful tool in determining how the transcriptional machinery of the cell is affected by external signals (e.g. drug treatment), or how cells differ between a healthy
Elisabeth G Klompenhouwer et al.
Nederlands tijdschrift voor geneeskunde, 157(8), A4231-A4231 (2013-02-22)
An 84-year-old male patient presented with a swelling on his forehead which had developed gradually over a period of three weeks after a fall. Ultrasound examination revealed a complex fluid collection with a yin-yang flow pattern (revealed by colour Doppler
Articles
Thrombin Factor IIa is an endolytic serine protease that selectively cleaves the Arg--Gly bonds of fibrinogen to form fibrin and release fibrinopeptides A and B.
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service