SRP3032
Enterokinase human
recombinant, expressed in CHO cells, ≥90% (SDS-PAGE), ≥90% (HPLC), suitable for cell culture
Sign Into View Organizational & Contract Pricing
All Photos(1)
About This Item
UNSPSC Code:
12352204
NACRES:
NA.32
Recommended Products
biological source
human
recombinant
expressed in CHO cells
Assay
≥90% (HPLC)
≥90% (SDS-PAGE)
form
lyophilized
mol wt
108.7 kDa
packaging
pkg of 50 μg
technique(s)
cell culture | mammalian: suitable
impurities
<0.1 EU/μg endotoxin, tested
color
white to off-white
UniProt accession no.
shipped in
wet ice
storage temp.
−20°C
Gene Information
human ... TMPRSS15(5651)
Related Categories
General description
Human Enterokinase is expressed as a linear 1019 amino acid polypeptide precursor glycoprotein. Proteolytic processing of this precursor generates the biologically active form of enterokinase, which consists of two polypeptide chains (heavy chain and light chain) held together by a single disulfide bond, resulting in formation of a biologically active heterodimer. The heavy chain consists of 784 amino acid residues, and the light consists of 235 amino acid residues. The mRNA is present in the proximal small intestine, and the protein is in enterocytes of the duodenum and proximal jejunum. The gene is mapped to human chromosome 21q21.
Biochem/physiol Actions
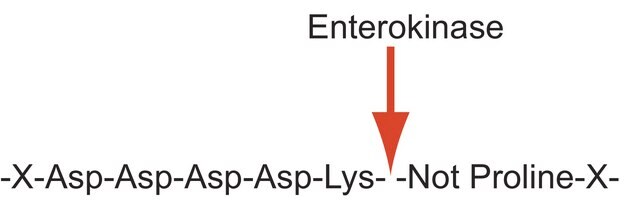
Proteases (also called proteolytic enzymes, peptidases, or proteinases) are enzymes that hydrolyze the amide bonds within proteins or peptides. Most proteases act in a specific manner, hydrolyzing bonds at or adjacent to specific residues or a specific sequence of residues contained within the substrate protein or peptide. Proteases play an important role in most diseases and biological processes including prenatal and postnatal development, reproduction, signal transduction, the immune response, various autoimmune and degenerative diseases, and cancer. Enterokinase sequentially cleaves carboxyl side of D-D-D-D-K. It is a serine protease which mainly activates trypsinogen.
Sequence
Heavy chain: LTIKESQRGA ALGQSHEARA TFKITSGVTY NPNLQDKLSV DFKVLAFDLQ QMIDEIFLSS NLKNEYKNSR VLQFENGSII VVFDLFFAQW VSDQNVKEEL IQGLEANKSS QLVTFHIDLN SVDILDKLTT TSHLATPGNV SIECLPGSSP CTDALTCIKA DLFCDGEVNC PDGSDEDNKM CATVCDGRFL LTGSSGSFQA THYPKPSETS VVCQWIIRVN QGLSIKLSFD DFNTYYTDIL DIYEGVGSSK ILRASIWETN PGTIRIFSNQ VTATFLIESD ESDYVGFNAT YTAFNSSELN NYEKINCNFE DGFCFWVQDL NDDNEWERIQ GSTFSPFTGP NFDHTFGNAS GFYISTPTGP GGRQERVGLL SLPLDPTLEP ACLSFWYHMY GENVHKLSIN ISNDQNMEKT VFQKEGNYGD NWNYGQVTLN ETVKFKVAFN AFKNKILSDI ALDDISLTYG ICNGSLYPEP TLVPTPPPEL PTDCGGPFEL WEPNTTFSST NFPNSYPNLA FCVWILNAQK GKNIQLHFQE FDLENINDVV EIRDGEEADS LLLAVYTGPG PVKDVFSTTN RMTVLLITND VLARGGFKAN FTTGYHLGIP EPCKADHFQC KNGECVPLVN LCDGHLHCED GSDEADCVRF FNGTTNNNGL VRFRIQSIWH TACAENWTTQ ISNDVCQLLG LGSGNSSKPI FSTDGGPFVK LNTAPDGHLI LTPSQQCLQD SLIRLQCNHK SCGKKLAAQD ITPK Light Chain: IVGGSNAKEG AWPWVVGLYY GGRLLCGASL VSSDWLVSAA HCVYGRNLEP SKWTAILGLH MKSNLTSPQT VPRLIDEIVI NPHYNRRRKD NDIAMMHLEF KVNYTDYIQP ICLPEENQVF PPGRNCSIAG WGTVVYQGTT ANILQEADVP LLSNERCQQQ MPEYNITENM ICAGYEEGGI DSCQGDSGGP LMCQENNRWF LAGVTSFGYK CALPNRPGVY ARVSRFTEWI QSFLH
Physical form
Lyophilized from 10 mM Sodium Phosphate, pH 7.5 + 1 mM Calcium Chloride.
Reconstitution
Centrifuge the vial prior to opening. Reconstitute in water to a concentration of 0.1-1.0 mg/ml. Do not vortex. This solution can be stored at 2-8°C for up to 1 week. For extended storage, it is recommended to store in working aliquots at -20°C to -80°C.
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Certificates of Analysis (COA)
Search for Certificates of Analysis (COA) by entering the products Lot/Batch Number. Lot and Batch Numbers can be found on a product’s label following the words ‘Lot’ or ‘Batch’.
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Mutations in the proenteropeptidase gene are the molecular cause of congenital enteropeptidase deficiency.
Holzinger A, et al.
American Journal of Human Genetics, 70, 20-25 (2002)
Biochemical characterization of human enteropeptidase light chain.
Gasparian ME, et al.
Biochemistry (Moscow), 71, 113-119 (2006)
An efficient method for expression in Escherichia coli and purification of the extracellular ligand binding domain of the human TGFbeta type II receptor.
Gasparian ME, et al.
Journal of Biotechnology, 148, 113-118 (2010)
Production and purification of recombinant enteropeptidase expressed in an insect-baculovirus cell system.
Azhar M and Somashekhar R
Preparative Biochemistry & Biotechnology, 45, 268-278 (2015)
Shenoy Rajesh Tulsidas et al.
Acta crystallographica. Section F, Structural biology and crystallization communications, 65(Pt 5), 533-535 (2009-05-02)
Serine proteases play a major role in host-pathogen interactions. The innate immune system is known to respond to invading pathogens in a nonspecific manner. The serine protease cascade is an essential component of the innate immune system of the horseshoe
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service