P0440
Pimaricin preparation
~2.5% (γ-irradiated Pimaricin), aqueous suspension
Synonym(s):
Pimaricin, Tennecetin, Natamycin preparation
About This Item
Recommended Products
form
aqueous suspension
concentration
~2.5% (γ-irradiated Pimaricin)
solubility
DMSO: soluble
density
1.0 g/mL at 20 °C (lit.)
antibiotic activity spectrum
fungi
yeast
Mode of action
cell membrane | interferes
storage temp.
2-8°C
SMILES string
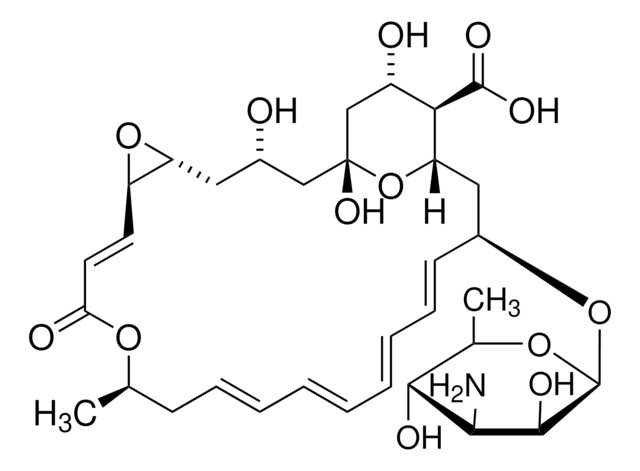
[H][C@]12C[C@@H](O[C@@H]3O[C@H](C)[C@@H](O)[C@H](N)[C@@H]3O)\C=C\C=C\C=C\C=C\C[C@@H](C)OC(=O)\C=C\[C@@]4([H])O[C@]4([H])C[C@H](O)C[C@](O)(C[C@H](O)[C@H]1C(O)=O)O2
InChI
1S/C33H47NO13/c1-18-10-8-6-4-3-5-7-9-11-21(45-32-30(39)28(34)29(38)19(2)44-32)15-25-27(31(40)41)22(36)17-33(42,47-25)16-20(35)14-24-23(46-24)12-13-26(37)43-18/h3-9,11-13,18-25,27-30,32,35-36,38-39,42H,10,14-17,34H2,1-2H3,(H,40,41)/b4-3+,7-5+,8-6+,11-9+,13-12+/t18-,19-,20+,21+,22+,23-,24-,25+,27-,28+,29-,30+,32+,33-/m1/s1
InChI key
NCXMLFZGDNKEPB-FFPOYIOWSA-N
Looking for similar products? Visit Product Comparison Guide
General description
Pimaricin is a polyene antifungal antibiotic produced by Streptomyces natalensis from soil near Pietermaritzburg, South Africa.1 Pimaricin has antimicrobial activity similar to that of nystatin. In addition, it is active against Trichomonas vaginalis. Pimaricin is used in the treatment of candidiasis, trichomoniasis, fungal keratitis and aspergillosis. It has also been used as a food additive in some countries. In some studies, it has been shown to decrease the amount of mold upon which the Dermatophagoides pteronyssinus (house-dust mite) is dependent.2
Application
Biochem/physiol Actions
Preparation Note
The product and any aqueous dilutions will be suspensions and should not be sterile filtered.
Storage and Stability
Other Notes
Storage Class Code
10 - Combustible liquids
WGK
WGK 2
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Personal Protective Equipment
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service