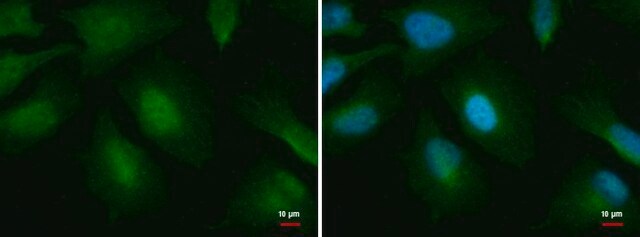
General description
OGT (O-linked N-acetylglucosamine (O GlcNAc) transferase) is a highly conserved cellular glucosyltransferase enzyme. It is an abundantly expressed protein. Its C-terminal contains the catalytic site, and the N-terminal contains multiple tetratricopeptide (TRP) repeats which constitute a long flexible scaffold.
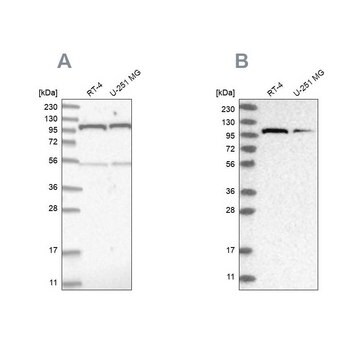
OGT (O-linked n-acetylglucosamine (O GlcNAc) transferase) enzyme is an homotrimer consisting of three subunits of 110 kDa each. The gene encoding OGT is mapped to human chromosome Xq13.1.
Immunogen
synthetic peptide corresponding to amino acids 740-756 of human O-GlcNAc transferase, conjugated to KLH via an N-terminal added cysteine residue.
Application
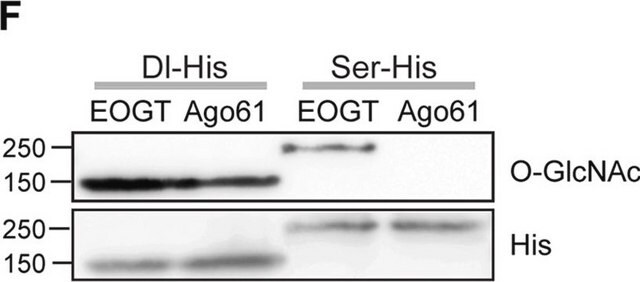
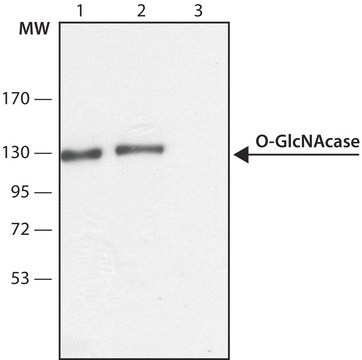
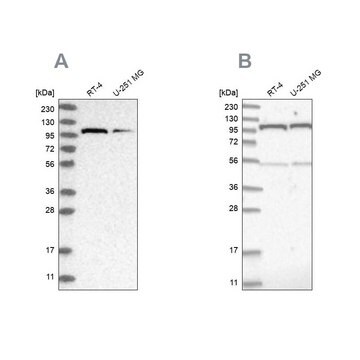
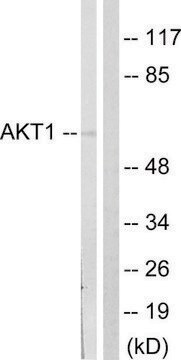
Anti-O-GlcNAc Transferase (DM-17) antibody produced in rabbit has also been used for immunoprecipitation.
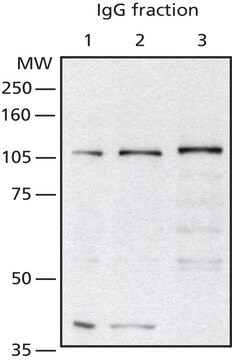
Anti-O-GlcNAc Transferase (DM-17) antibody produced in rabbit has been used for Western Blotting.
Biochem/physiol Actions
OGT (O-linked N-acetylglucosamine (O GlcNAc) transferase) is responsible for the O-linked addition of a single N-acetylglucosamine (GlcNAc) moiety to Ser and Thr residues during posttranslational modifications of nuclear and cytoplasmic proteins. It is, therefore, indirectly implicated in intracellular signaling by modulating the activity, stability, localization, and interactions of a variety of proteins. It promotes herpes simplex virus (HSV) replication, as it proteolytically cleave HCF-1 (host cell factor 1) transcriptional regulator responsible for transactivation of the immediate-early genes of HSV. In idiopathic pulmonary arterial hypertension (IPAH), this protein is responsible for alteration in glycosylation and proliferation of pulmonary artery smooth muscle cells (PASMCs).
The O-GlcNAcylation of intracellular proteins can occur on phosphorylation sites, and have been implicated in the regulation of gene transcription, diabetes, and neurological processes. O-GlcNAc modifications of nucleocytoplasmic proteins may serve as a negative feedback system for insulin signaling.(3,4) It is widely accepted that modification of serine and threonine residues by O-GlcNAc transferase is involved in the control of transcription, either by controlling elongation by RNA polymerase II or by targeting transcription factors to promoters. Growing evidences show that O-GlcNAc is involved in repressing transcription. OGT was also shown to interact with an histone deacetylase complex by binding to the correpressor Sin3A. This interaction leads to the repression of transcription after transcription factors and RNA polymerase II are modified by addition of O-GlcNAc. Strikingly, O-GlcNAc modification reversibly inhibits proteosomal function in an ubiquitin independent fashion.
Physical form
Solution in 0.01 M phosphate buffered saline, pH 7.4, and 15 mM sodium azide.
Disclaimer
Unless otherwise stated in our catalog or other company documentation accompanying the product(s), our products are intended for research use only and are not to be used for any other purpose, which includes but is not limited to, unauthorized commercial uses, in vitro diagnostic uses, ex vivo or in vivo therapeutic uses or any type of consumption or application to humans or animals.