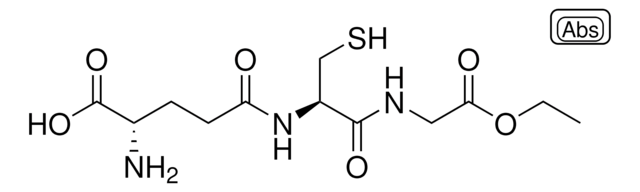
M4139
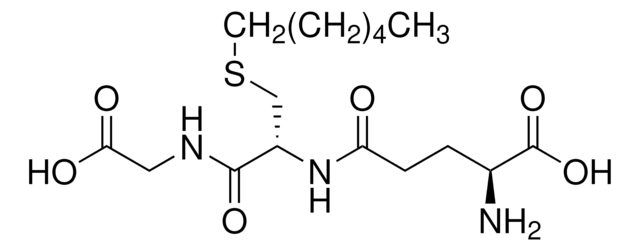
S-Methylglutathione
>98% (TLC), suitable for cell culture
Synonym(s):
L-γ-glutamyl-S-methyl-L-cysteinyl-glycine
Sign Into View Organizational & Contract Pricing
All Photos(3)
About This Item
Empirical Formula (Hill Notation):
C11H19N3O6S
CAS Number:
Molecular Weight:
321.35
MDL number:
UNSPSC Code:
12352209
PubChem Substance ID:
NACRES:
NA.26
Recommended Products
Product Name
S-Methylglutathione,
Assay
>98% (TLC)
Quality Level
form
powder
technique(s)
cell culture | mammalian: suitable
color
white
storage temp.
2-8°C
SMILES string
CSCC(NC(=O)CCC(N)C(O)=O)C(=O)NCC(O)=O
InChI
1S/C11H19N3O6S/c1-21-5-7(10(18)13-4-9(16)17)14-8(15)3-2-6(12)11(19)20/h6-7H,2-5,12H2,1H3,(H,13,18)(H,14,15)(H,16,17)(H,19,20)
InChI key
QTQDDTSVRVWHMO-UHFFFAOYSA-N
Related Categories
Biochem/physiol Actions
S-methylglutathione is a methionine containing peptide and glyoxylase inhibitor.
Substrates
Useful as a glyoxylase inhibitor.
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
M F Phillips et al.
The Biochemical journal, 294 ( Pt 1), 57-62 (1993-08-15)
Mouse liver glutathione S-transferase YfYf (Pi class) reacts with [14C]ethacrynic acid to form a covalent adduct with a stoichiometry of 1 mol per mol of subunit. Proteolytic digestion of the enzyme-[14C]ethacrynic acid adduct with V8 protease produced an 11 kDa
M Müller et al.
Archives of toxicology, 74(12), 760-767 (2001-04-18)
Glutathione-S-transferase T1 (GSTT1-1) is a major isoenzyme for the biotransformation of halomethanes. The enzyme activity is located, among other places, in human liver and erythrocytes and is subject to a genetic polymorphism. Metabolism of the halomethanes via GSTT1-1 yields S-methylglutathione
Y Manabe et al.
Chemical senses, 25(2), 173-180 (2000-04-26)
Tentacle ball formation (TBF) in Hydra elicited by S-methylglutathione (GSM) was modulated by a number of biologically active peptides. Hydra fed on Artemia, which had been hatched in a common salt solution supplemented with LiCl and ZnCl(2), easily induced TBF
K Hanai
Comparative biochemistry and physiology. Part A, Molecular & integrative physiology, 119(1), 333-339 (2001-03-20)
Within minutes, brief treatment with trypsin potentiated tentacle ball formation in Hydra japonica, a new behavioral response to reduced glutathione. With the potentiation of this behavioral response, new glutathione-binding proteins were immediately detected after the trypsin treatment of live Hydra
O K Vatamaniuk et al.
The Journal of biological chemistry, 275(40), 31451-31459 (2000-05-16)
The dependence of phytochelatin synthase (gamma-glutamylcysteine dipeptidyltranspeptidase (PCS), EC ) on heavy metals for activity has invariably been interpreted in terms of direct metal binding to the enzyme. Here we show, through analyses of immunopurified, recombinant PCS1 from Arabidopsis thaliana
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service