All Photos(1)
About This Item
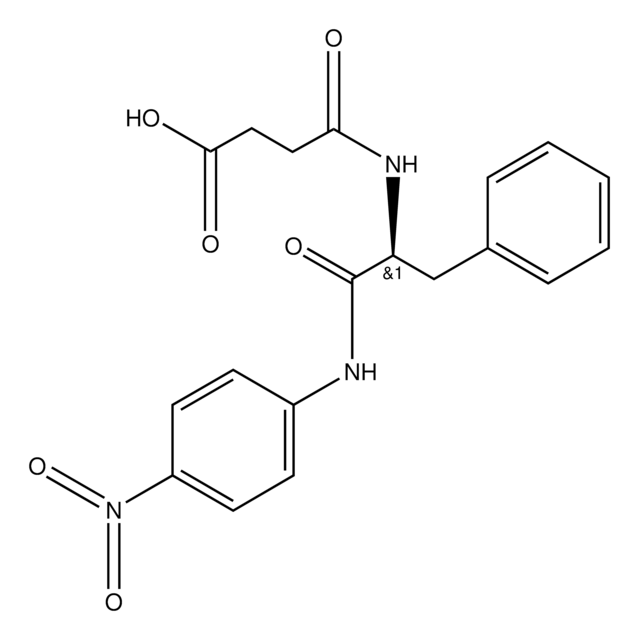
Empirical Formula (Hill Notation):
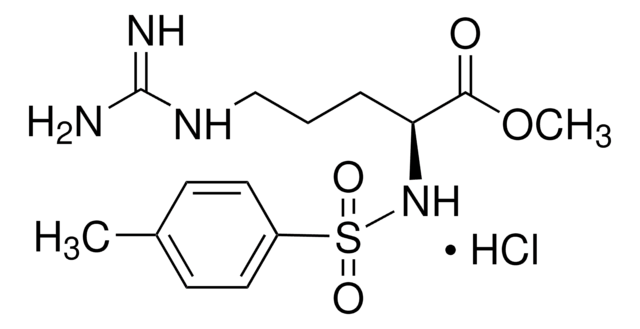
C24H29N5O7
CAS Number:
Molecular Weight:
499.52
MDL number:
UNSPSC Code:
12352204
PubChem Substance ID:
NACRES:
NA.32
Recommended Products
Assay
≥98% (TLC)
form
powder
solubility
methanol: 50 mg/mL, clear, colorless to faintly yellow
storage temp.
−20°C
SMILES string
CC(C)CC(NC(=O)CNC(=O)CNC(=O)OCc1ccccc1)C(=O)Nc2ccc(cc2)N(=O)=O
InChI
1S/C24H29N5O7/c1-16(2)12-20(23(32)27-18-8-10-19(11-9-18)29(34)35)28-22(31)14-25-21(30)13-26-24(33)36-15-17-6-4-3-5-7-17/h3-11,16,20H,12-15H2,1-2H3,(H,25,30)(H,26,33)(H,27,32)(H,28,31)
InChI key
IHRYETONKBXGOF-UHFFFAOYSA-N
Substrates
A sensitive chromogenic substrate for subtilisins and neutral endopeptidases.
Storage Class Code
13 - Non Combustible Solids
WGK
WGK 3
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
A new chromogenic substrate for subtilisin.
L A Lyublinskaya et al.
Analytical biochemistry, 62(2), 371-376 (1974-12-01)
Degradation of bradykinin by isolated neutral endopeptidases of brain and pituitary.
S Wilk et al.
Biochemical and biophysical research communications, 90(1), 1-6 (1979-09-12)
Ajay Kumar Shaw et al.
Journal of photochemistry and photobiology. B, Biology, 86(3), 199-206 (2006-11-18)
Enzymatic activity of a proteolytic enzyme Subtilisin Carlsberg (SC) in anionic sodium dodecyl sulfate (SDS) micellar medium has been explored and found to be retarded compared to that in bulk buffer. Circular dichroism (CD) study reveals that SDS, which is
J R Arbona et al.
Journal of animal science, 71(12), 3301-3306 (1993-12-01)
Within 1 h after slaughter, two 10-g samples of longissimus muscle were obtained from four crossbred beef cattle. Samples were homogenized in three or six volumes of extraction solution that consisted of 50 mM Tris base, 10 mM EDTA, and
Evidence that pituitary cation-sensitive neutral endopeptidase is a multicatalytic protease complex.
S Wilk et al.
Journal of neurochemistry, 40(3), 842-849 (1983-03-01)
Pituitary cation-sensitive neutral endopeptidase splits peptide bonds on the carboxyl side of hydrophobic amino acids (chymotrypsin-like activity), basic amino acids (trypsin-like activity), and acidic amino acids (peptidyl-glutamyl-peptide bond hydrolyzing activity). All three activities copurify, are inhibited by cations, and reside
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service