All Photos(2)
About This Item
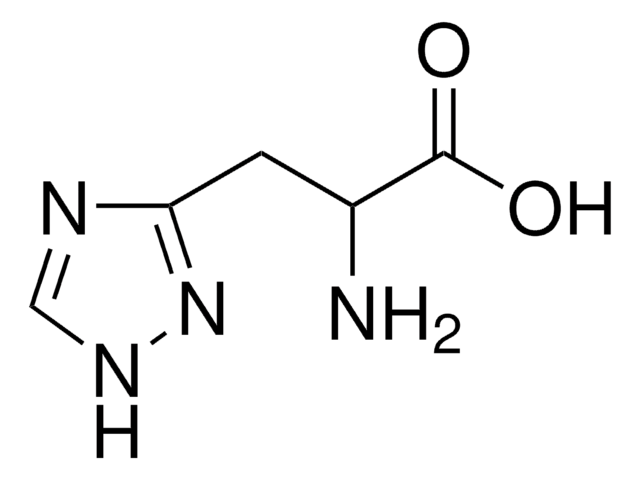
Empirical Formula (Hill Notation):
C10H11N3O2 · H2O
CAS Number:
Molecular Weight:
223.23
MDL number:
UNSPSC Code:
12352209
PubChem Substance ID:
NACRES:
NA.26
Recommended Products
product name
DL-7-Azatryptophan hydrate,
Assay
≥98% (TLC)
form
powder
color
white to off-white
storage temp.
−20°C
SMILES string
O.NC(Cc1c[nH]c2ncccc12)C(O)=O
InChI
1S/C10H11N3O2.H2O/c11-8(10(14)15)4-6-5-13-9-7(6)2-1-3-12-9;/h1-3,5,8H,4,11H2,(H,12,13)(H,14,15);1H2
InChI key
PXDRHYQAIUZKHN-UHFFFAOYSA-N
Biochem/physiol Actions
DL-7-Azatryptophan is a racemic mixture of D- and L-7-azatryptophan which together with L-tryptophan is a synergistic inducer of tryptophan oxygenase of Pseudomonas acidovorans. DL-7-Azatryptophan inhibits photosynthetic carbon assimilation, photosynthetic oxygen evolution and nitrogen metabolism in Anabaena sp. Strain 1F, a marine filamentous, heterocystous cyanobacterium.
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Certificates of Analysis (COA)
Search for Certificates of Analysis (COA) by entering the products Lot/Batch Number. Lot and Batch Numbers can be found on a product’s label following the words ‘Lot’ or ‘Batch’.
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Julie M G Rogers et al.
Analytical biochemistry, 399(2), 182-189 (2009-12-29)
Fluorescence resonance energy transfer (FRET) provides a powerful means to study protein conformational changes. However, the incorporation of an exogenous FRET pair into a protein could lead to undesirable structural perturbations of the native fold. One of the viable strategies
Arnaldo L Serrano et al.
The journal of physical chemistry. B, 116(35), 10631-10638 (2012-08-16)
The villin headpiece subdomain (HP35) has become one of the most widely used model systems in protein folding studies, due to its small size and ultrafast folding kinetics. Here, we use HP35 as a test bed to show that the
P Soumillion et al.
Protein engineering, 8(5), 451-456 (1995-05-01)
The phage lambda lysozyme (lambda L) contains four tryptophans. These have been efficiently replaced by 7-azatryptophan (7aW) through biosynthetic incorporation into the overexpressed protein. Comparative analysis of the effect of temperature or pH on the fluorescence of the wild-type lambda
Fernando Formaggio et al.
Advances in experimental medicine and biology, 527, 731-737 (2004-06-23)
Aal and 7-Atrp, quasi-isosteric with Trp, have been inserted together with a TOAC residue in two 3(10)-helical, model hexapeptides. The interaction of photoexcited AA1 and 7-Atrp with the nitroxide group of TOAC was investigated by time resolved EPR. Both peptides
Vasant Muralidharan et al.
Journal of the American Chemical Society, 126(43), 14004-14012 (2004-10-28)
An integrated approach is described that allows the domain-specific incorporation of optical probes into large recombinant proteins. The strategy is the combination of two existing techniques, expressed protein ligation (EPL) and in vivo amino acid replacement of tryptophans with tryptophan
Chromatograms
application for HPLCOur team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service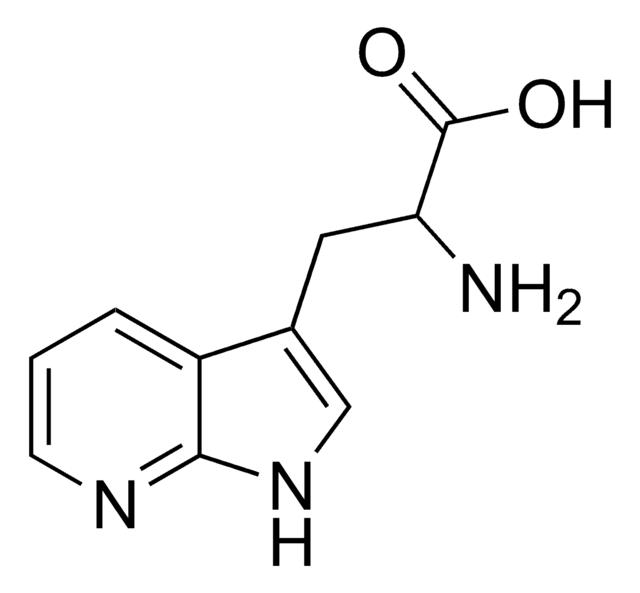
![1,2,3,4-Tetrahydro-9H-pyrido[3,4-b]indole 98%](/deepweb/assets/sigmaaldrich/product/structures/181/460/3d58bc34-1b5c-4295-bbac-3b52085670e8/640/3d58bc34-1b5c-4295-bbac-3b52085670e8.png)