D152927
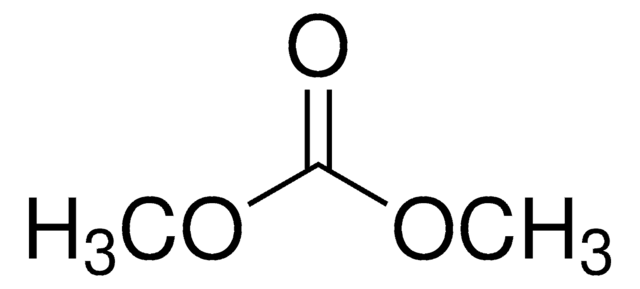
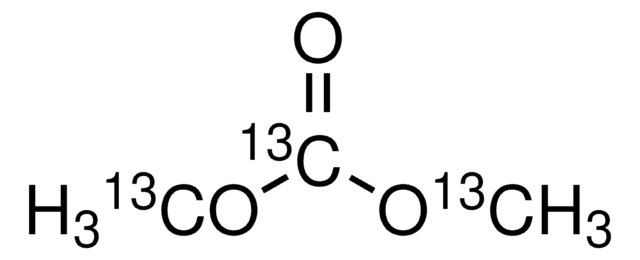
Dimethyl carbonate
ReagentPlus®, 99%
Synonym(s):
Carbonic acid dimethyl ester
About This Item
Recommended Products
vapor density
3.1 (vs air)
Quality Level
vapor pressure
18 mmHg ( 21.1 °C)
product line
ReagentPlus®
Assay
99%
form
liquid
greener alternative product characteristics
Less Hazardous Chemical Syntheses
Safer Solvents and Auxiliaries
Design for Degradation
Learn more about the Principles of Green Chemistry.
sustainability
Greener Alternative Product
refractive index
n20/D 1.368 (lit.)
bp
90 °C (lit.)
mp
2-4 °C (lit.)
density
1.069 g/mL at 25 °C (lit.)
greener alternative category
, Aligned
SMILES string
O=C(OC)OC
InChI
1S/C3H6O3/c1-5-3(4)6-2/h1-2H3
InChI key
IEJIGPNLZYLLBP-UHFFFAOYSA-N
Looking for similar products? Visit Product Comparison Guide
General description
We are committed to bringing you Greener Alternative Products, which adhere to one or more of The 12 Principles of Greener Chemistry. This product is a Greener alternative to conventional solvents and chemicals. Click here for more information.
Application
- Methyl phenyl carbonate by transesterification with phenol.
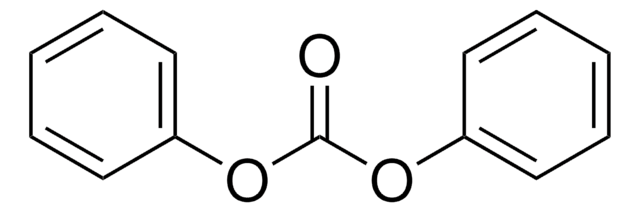
- Diphenyl carbonate by transesterification with methyl phenyl carbonate.
- Methyl carbamates, a raw material for isocyanate synthesis.
- Tetramethoxysilane by reacting with silica at 550-600K.
Features and Benefits
Legal Information
Signal Word
Danger
Hazard Statements
Precautionary Statements
Hazard Classifications
Flam. Liq. 2
Storage Class Code
3 - Flammable liquids
WGK
WGK 1
Flash Point(F)
60.8 °F - closed cup
Flash Point(C)
16 °C - closed cup
Personal Protective Equipment
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Articles
Research and development of solid-state lithium fast-ion conductors is crucial because they can be potentially used as solid electrolytes in all-solid-state batteries, which may solve the safety and energy-density related issues of conventional lithium-ion batteries that use liquid (farmable organic) electrolytes.
Related Content
Why should you have to choose between solvents that are ecological and those that are reliable? Enjoy both at once with our biorenewable and greener solutions. Cyrene™ solvent is a new dipolar aprotic alternative to common REACH restricted solvents, such as N methyl-2-pyrrolidone (NMP) and Dimethylformamide (DMF).
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service