About This Item
Recommended Products
grade
analytical standard
Quality Level
product line
PESTANAL®
shelf life
limited shelf life, expiry date on the label
technique(s)
HPLC: suitable
gas chromatography (GC): suitable
application(s)
agriculture
environmental
format
neat
storage temp.
2-8°C
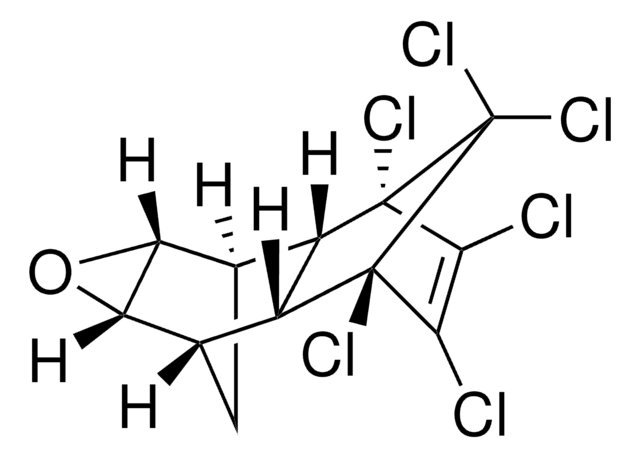
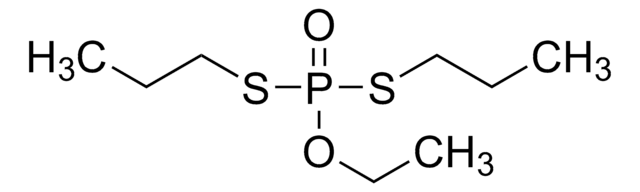
SMILES string
CCOP(=S)(OCC)SCCSCC
InChI
1S/C8H19O2PS3/c1-4-9-11(12,10-5-2)14-8-7-13-6-3/h4-8H2,1-3H3
InChI key
DOFZAZXDOSGAJZ-UHFFFAOYSA-N
Looking for similar products? Visit Product Comparison Guide
Application
Legal Information
Signal Word
Danger
Hazard Statements
Precautionary Statements
Hazard Classifications
Acute Tox. 1 Dermal - Acute Tox. 2 Oral - Aquatic Acute 1 - Aquatic Chronic 1
Storage Class Code
6.1A - Combustible acute toxic Cat. 1 and 2 / very toxic hazardous materials
WGK
WGK 3
Flash Point(F)
271.4 °F
Flash Point(C)
133 °C
Personal Protective Equipment
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Protocols
analytical standard; Tokuthion, technical grade, pkg of 50 mg; Crotoxyphos; Phorate; Azinphos-ethyl; Diazinon; Azinphos-methyl; Demeton-O; Dimethoate; Chlorpyrifos-methyl
Chlorobenzilate; 4-Aminobiphenyl; 2-Fluorobiphenyl; N-Nitrosopyrrolidine; 1,2,4,5-Tetrachlorobenzene; 3-Methylcholanthrene; Phenacetin
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service