840435P
Avanti
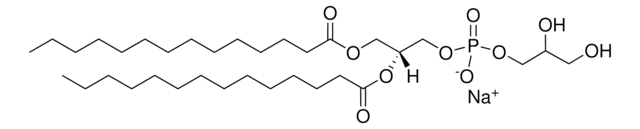
12:0 PG
Avanti Research™ - A Croda Brand
Synonym(s):
1,2-didodecanoyl-sn-glycero-3-phospho-(1′racglycerol) (sodium salt); DLPG; PG(12:0/12:0)
About This Item
Recommended Products
description
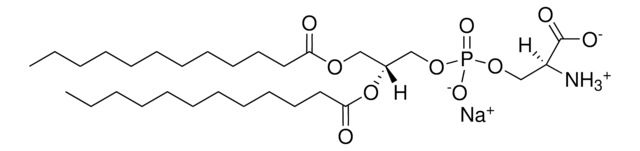
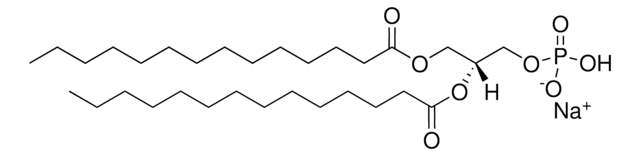
1,2-dilauroyl-sn-glycero-3-phospho-(1′-rac-glycerol) (sodium salt)
Assay
>99% (TLC)
form
powder
packaging
pkg of 1 × 200 mg (840435P-200mg)
pkg of 1 × 25 mg (840435P-25mg)
manufacturer/tradename
Avanti Research™ - A Croda Brand
lipid type
cardiolipins
phospholipids
shipped in
dry ice
storage temp.
−20°C
SMILES string
[Na+].[P](=O)([O-])(OC[C@H](OC(=O)CCCCCCCCCCC)COC(=O)CCCCCCCCCCC)OCC(O)CO
InChI
1S/C30H59O10P.Na/c1-3-5-7-9-11-13-15-17-19-21-29(33)37-25-28(26-39-41(35,36)38-24-27(32)23-31)40-30(34)22-20-18-16-14-12-10-8-6-4-2;/h27-28,31-32H,3-26H2,1-2H3,(H,35,36);/q;+1/p-1/t27?,28-;/m1./s1
InChI key
CIRSYGHBBDDURM-AMRYRHMLSA-M
General description
Application
- in the preparation of liposomes for PagP folding assays
- as a component in pyrene labeleld liposomes
- to construct liposome/lipid vesicle
Biochem/physiol Actions
Packaging
Legal Information
also commonly purchased with this product
Storage Class Code
11 - Combustible Solids
Choose from one of the most recent versions:
Certificates of Analysis (COA)
It looks like we've run into a problem, but you can still download Certificates of Analysis from our Documents section.
If you need assistance, please contact Customer Support.
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Articles
The critical micelle concentration (CMC) can be approximately defined as the lipid monomer concentration at which appreciable amounts (>5% of total) of micellar aggregates first begin to appear in the equilibrium: nM1<=>Mn
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service