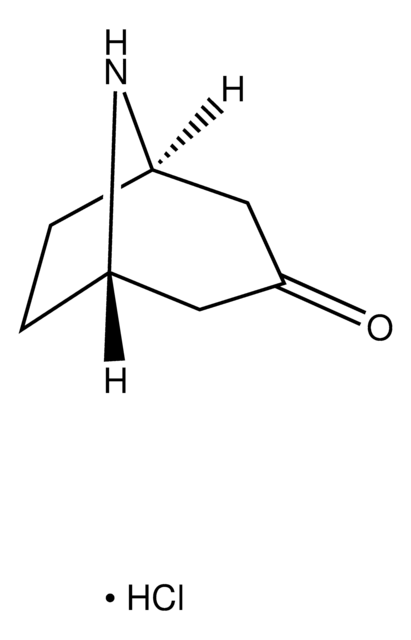
T89605
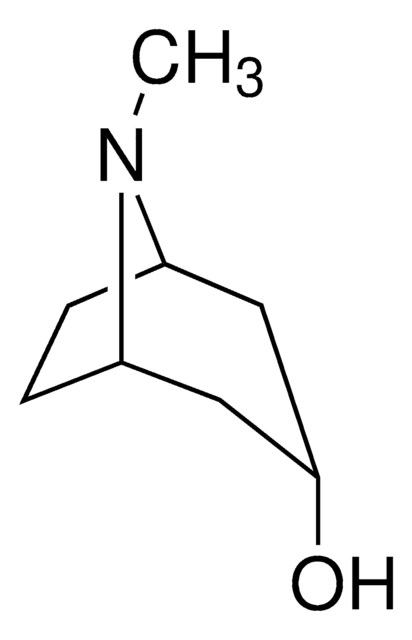
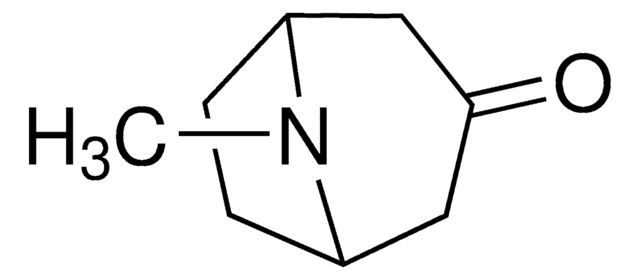
Tropinone
99%
Synonym(s):
8-Methyl-8-azabicyclo[3.2.1]octan-3-one
Sign Into View Organizational & Contract Pricing
All Photos(2)
About This Item
Empirical Formula (Hill Notation):
C8H13NO
CAS Number:
Molecular Weight:
139.19
Beilstein:
2329
EC Number:
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Assay
99%
bp
113 °C/25 mmHg (lit.)
mp
40-44 °C (lit.)
storage temp.
2-8°C
SMILES string
CN1[C@@H]2CC[C@H]1CC(=O)C2
InChI
1S/C8H13NO/c1-9-6-2-3-7(9)5-8(10)4-6/h6-7H,2-5H2,1H3/t6-,7+
InChI key
QQXLDOJGLXJCSE-KNVOCYPGSA-N
Looking for similar products? Visit Product Comparison Guide
Related Categories
Signal Word
Warning
Hazard Statements
Precautionary Statements
Hazard Classifications
Acute Tox. 4 Inhalation - Acute Tox. 4 Oral
Storage Class Code
11 - Combustible Solids
WGK
WGK 1
Flash Point(F)
194.0 °F - closed cup
Flash Point(C)
90 °C - closed cup
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Enantioselective synthesis of unnatural (S)-(+)-cocaine.
J C Lee et al.
The Journal of organic chemistry, 65(15), 4773-4775 (2000-08-26)
E Leete
Planta medica, 56(4), 339-352 (1990-08-01)
Recent work on the biosynthesis of the tropane moiety of cocaine, hyoscamine, scopolamine, and related alkaloids is reviewed. Revision of the generally accepted biosynthetic pathway to these alkaloids is now proposed in the light of new discoveries. New information on
Franklin A Davis et al.
Organic letters, 11(7), 1647-1650 (2009-03-13)
Sulfinimine-derived, enantiopure N-sulfinyl beta-amino ketone ketals on hydrolysis give dehydropyrrolidine ketones that on treatment with (Boc)(2)O/DMAP afford substituted tropinones in good yield.
Roger L Papke et al.
Neuroscience letters, 378(3), 140-144 (2005-03-23)
The alpha7 nicotinic acetylcholine receptor (nAChR)-selective partial agonist tropisetron is a conjugate of an indole and a tropane group. We tested compounds structurally related to either the indole or tropane domains of tropisetron on oocytes expressing human alpha7. alpha4beta2, or
Yvonne Scholl et al.
Phytochemistry, 62(3), 325-332 (2003-03-07)
Calystegines are nortropane alkaloids bearing between three and five hydroxyl groups in various positions. [15N]Tropinone was administered to root cultures of Calystegia sepium and the incorporation into calystegines was followed. Increase of label in calystegines was measured by one-dimensional 15N
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service