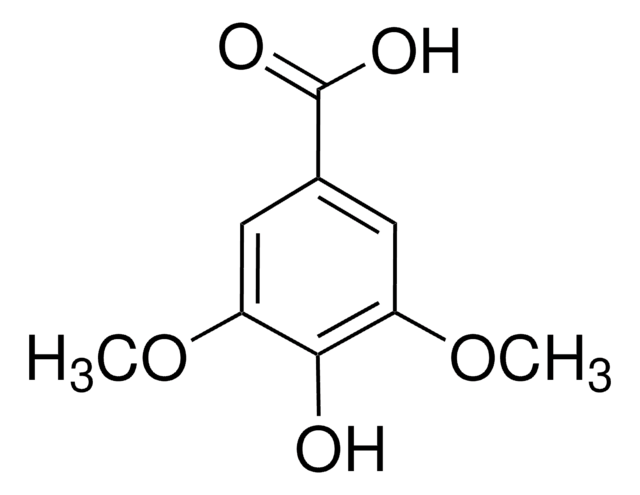
H23007
trans-3-Hydroxycinnamic acid
99%
Synonym(s):
(E)-3-(3-Hydroxyphenyl)acrylic acid, m-Coumaric acid
Sign Into View Organizational & Contract Pricing
All Photos(1)
About This Item
Linear Formula:
HOC6H4CH=CHCO2H
CAS Number:
Molecular Weight:
164.16
Beilstein:
2084229
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Quality Level
Assay
99%
mp
193-195 °C (lit.)
SMILES string
OC(/C=C/C1=CC=CC(O)=C1)=O
InChI
1S/C9H8O3/c10-8-3-1-2-7(6-8)4-5-9(11)12/h1-6,10H,(H,11,12)/b5-4+
InChI key
KKSDGJDHHZEWEP-SNAWJCMRSA-N
Looking for similar products? Visit Product Comparison Guide
Signal Word
Warning
Hazard Statements
Precautionary Statements
Hazard Classifications
Eye Irrit. 2 - Skin Irrit. 2 - STOT SE 3
Target Organs
Respiratory system
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Alexis Eugene et al.
Biotechnology for biofuels, 13(1), 202-202 (2020-12-12)
Arabinoxylan in grass cell walls is acylated to varying extents by ferulate and p-coumarate at the 5-hydroxy position of arabinosyl residues branching off the xylan backbone. Some of these hydroxycinnamate units may then become involved in cell wall radical coupling
J Cabanes et al.
Biochimica et biophysica acta, 790(2), 101-107 (1984-10-23)
The inhibition by m-coumaric acid of oxidation of L-dopa by epidermis tyrosinase (monophenol,dihydroxy-L-phenylalanine:oxygen oxidoreductase, EC 1.14.18.1) is characterized by a prolonged transient phase. Kinetic data correspond to that for a postulated mechanism that involves rapid formation of a reduced enzyme-m-coumaric
Seigo Baba et al.
Life sciences, 75(2), 165-178 (2004-05-04)
Rosmarinic acid (RA) is contained in various Lamiaceae herbs used commonly as culinary herbs. Although RA has various potent physiological actions, little is known on its bioavailability. We therefore investigated the absorption and metabolism of orally administered RA in rats.
Antonia Nostro et al.
Natural product research, 26(22), 2132-2136 (2011-10-22)
This study reported the antimicrobial activity and phenolic content of natural site and micropropagated Limonium avei (De Not.) Brullo & Erben inflorescences. The minimum inhibitory concentration (MIC) and minimum bactericidal concentration (MBC) of ethanolic extracts were determined according to the
Yutaka Konishi et al.
Journal of agricultural and food chemistry, 52(21), 6418-6424 (2004-10-14)
It was previously reported that m-coumaric acid, m-hydroxyphenylpropionic acid (mHPP), and 3,4-dihydroxyphenylpropionic acid (DHPP) are major metabolites of ingested caffeic acid formed by gut microflora and would be transported by the monocarboxylic acid transporter (MCT). We have directly measured their
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service