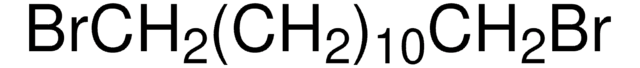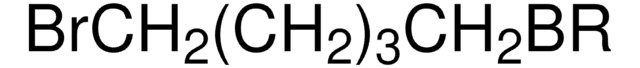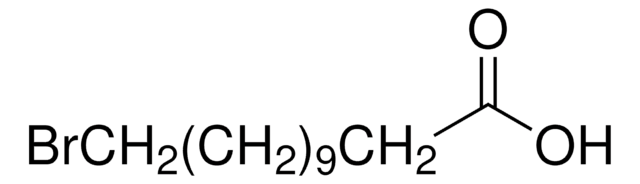D39800
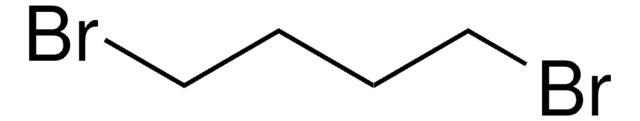
1,10-Dibromodecane
97%
Synonym(s):
Decamethylene dibromide
Sign Into View Organizational & Contract Pricing
All Photos(2)
About This Item
Linear Formula:
Br(CH2)10Br
CAS Number:
Molecular Weight:
300.07
Beilstein:
506156
EC Number:
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Quality Level
Assay
97%
form
crystals
refractive index
n20/D 1.4912 (lit.)
bp
160 °C/15 mmHg (lit.)
mp
25-27 °C (lit.)
density
1.335 g/mL at 25 °C (lit.)
SMILES string
BrCCCCCCCCCCBr
InChI
1S/C10H20Br2/c11-9-7-5-3-1-2-4-6-8-10-12/h1-10H2
InChI key
GTQHJCOHNAFHRE-UHFFFAOYSA-N
Looking for similar products? Visit Product Comparison Guide
Related Categories
General description
1,10-Dibromodecane, also known as Decamethylene dibromide, is an aromatic and aliphatic monomer, often utilized in the polymerization reaction to synthesize high molecular weight polysulfides through polycondensation.
Application
1,10-Dibromodecane is used:
- as a reactant in the Wurtz-type reaction during the synthesis of polyethylene
- Construction of pillar[4]arene[1]quinone-1,10-dibromodecane pseudorotaxanes in solution and in the solid state.: This research highlights the application of 1,10-Dibromodecane in constructing complex molecular structures known as pseudorotaxanes. The study focuses on the synthesis and stabilization of these structures both in solution and solid states, providing a foundational technique for developing advanced materials in chemical engineering, pharmaceutical synthesis, and high-performance materials within industrial chemical manufacturing sectors. This breakthrough offers potential pathways for new drug delivery systems and smart materials based on the unique properties of these supramolecular assemblies (Sheng et al., 2020).
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Flash Point(F)
233.6 °F - closed cup
Flash Point(C)
112 °C - closed cup
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Rashid Nazir et al.
ACS applied materials & interfaces, 12(6), 7639-7649 (2020-01-24)
Design and synthesis of nanostructured responsive gels have attracted increasing attention, particularly in the biomedical domain. Polymer chain configurations and nanodomain sizes within the network can be used to steer their functions as drug carriers. Here, a catalyst-free facile one-step
Siyang He et al.
Molecules (Basel, Switzerland), 26(4) (2021-02-11)
Diels-Alder reactions on the surface of nanoparticles allow a thermoreversible functionalization of the nanosized building blocks. We report the synthesis of well-defined magnetite nanoparticles by thermal decomposition reaction and their functionalization with maleimide groups. Attachment of these dienophiles was realized
Elżbieta Luboch et al.
Journal of inclusion phenomena and macrocyclic chemistry, 83(3-4), 321-334 (2015-11-10)
The article presents the synthesis of novel 13- and 16-membered azobenzocrown derivatives with peripheral thiol moieties and preliminary studies assessing their possible application in plasmonic sensors based on gold nanoparticles. The effect of the length of the chain connecting the
Xin Jiang et al.
Cell, 183(1), 258-268 (2020-08-30)
Plasmodium species, the causative agent of malaria, rely on glucose for energy supply during blood stage. Inhibition of glucose uptake thus represents a potential strategy for the development of antimalarial drugs. Here, we present the crystal structures of PfHT1, the
Global Trade Item Number
| SKU | GTIN |
|---|---|
| D39800-100G | 4061833563762 |
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service