All Photos(3)
About This Item
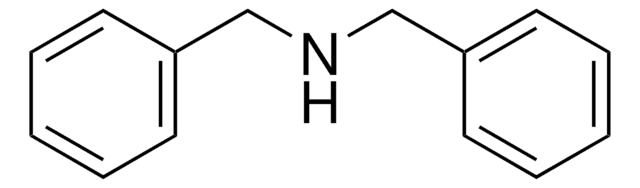
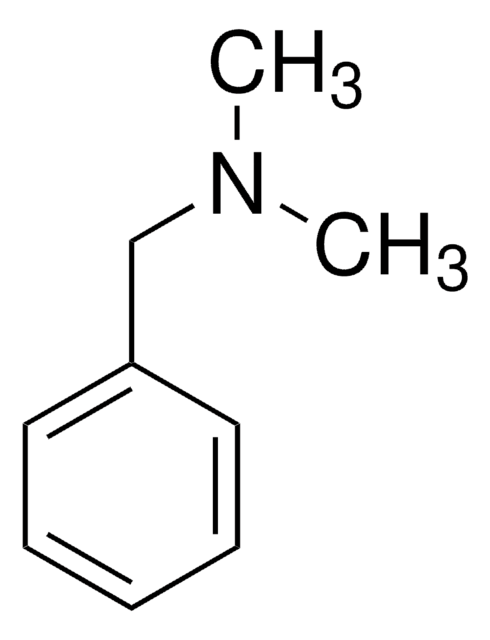
Linear Formula:
(C6H5CH2)3N
CAS Number:
Molecular Weight:
287.40
Beilstein:
2214682
EC Number:
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Assay
≥99.0% (NT)
form
powder
mp
91-94 °C (lit.)
functional group
amine
phenyl
SMILES string
C(N(Cc1ccccc1)Cc2ccccc2)c3ccccc3
InChI
1S/C21H21N/c1-4-10-19(11-5-1)16-22(17-20-12-6-2-7-13-20)18-21-14-8-3-9-15-21/h1-15H,16-18H2
InChI key
MXHTZQSKTCCMFG-UHFFFAOYSA-N
Looking for similar products? Visit Product Comparison Guide
Application
Tribenzylamine (TBA) is a tertiary amine which can be used :
TBA can also undergo debenzylation in the presence of ceric ammonium nitrate (CAN) to form dibenzylamine.
- As a nitrogen group source for the reactions involving C−N bond formation.
- For the synthesis of imine i.e. N−benzylidene benzylamine by aerobic oxidative condensation.
- As an extractant for the separation and determination of Cr(VI) and Cr(III) from wastewater.
TBA can also undergo debenzylation in the presence of ceric ammonium nitrate (CAN) to form dibenzylamine.
Signal Word
Warning
Hazard Statements
Precautionary Statements
Hazard Classifications
Skin Sens. 1
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Flash Point(F)
399.2 °F - closed cup
Flash Point(C)
204 °C - closed cup
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Copper-Catalyzed Oxidative Amination of Benzoxazoles via C- H and C- N Bond Activation: A New Strategy for Using Tertiary Amines as Nitrogen Group Sources.
Guo S, et al.
Organic Letters, 13(3), 522-525 (2010)
Chemoselective oxidative debenzylation of tertiary N-benzyl amines.
Bull SD, et al.
Chemical Communications (Cambridge, England), 165(5), 337-338 (2000)
Highly active and selective gold catalysts for the aerobic oxidative condensation of benzylamines to imines and one-pot, two-step synthesis of secondary benzylamines.
Grirrane A, et al.
J. Catal., 264(2), 138-144 (2009)
Céline Burnier et al.
Talanta, 192, 135-141 (2018-10-24)
Nowadays, Gas Chromatography Mass Spectrometry (GC-MS) is mainly used in forensic sciences but suffers from limitations when the analysed compounds are thermally instable as it is the case for THC-A (Tetrahydrocannabinolic Acid) which is converted into Δ9-THC (Δ9-Tetrahydrocannabinol) that subsequently
Yan Qu et al.
Zeitschrift fur Naturforschung. C, Journal of biosciences, 58(9-10), 640-642 (2003-10-28)
Two compounds, (p-methoxyphenyl) diphenylmethanol (1) and tribenzylamine (2), were isolated from Humulus lupulus. Their structures were established on the basis of spectral evidence (MS, IR, NMR, HMBC, HMQC, 1H-1H COSY experiments). Compounds 1 and 2 were found as natural products
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service