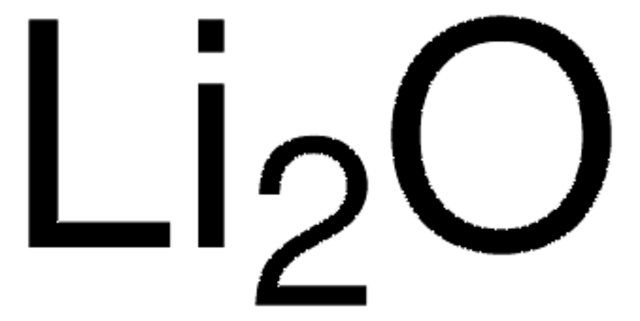669431
Lithium fluoride
Precipitated, 99.995%
Synonym(s):
Fluorolithium
About This Item
Recommended Products
Assay
99.995%
form
powder
impurities
≤55.0 ppm Trace Metal Analysis
mp
845 °C (lit.)
density
2.64 g/mL at 25 °C (lit.)
SMILES string
[Li+].[F-]
InChI
1S/FH.Li/h1H;/q;+1/p-1
InChI key
PQXKHYXIUOZZFA-UHFFFAOYSA-M
Looking for similar products? Visit Product Comparison Guide
General description
Application
- To fabricate an insulating layer in AC-driven organic light-emitting diodes(OLEDs). LiF provides excellent capacitance and high stability to the insulating layers in AC devices.
- As a precursor to synthesize Eu2+/Ce3+ doped barium lithium fluoride phosphors by solvothermal method.
- To fabricate LiF thin film coating on the lithium anodes to enhance their stability and coulombic efficiency.
- As an additive to enrich and enhance the performance of solidelectrolyte interface(SEI) in Li metal batteries.
Signal Word
Danger
Hazard Statements
Precautionary Statements
Hazard Classifications
Acute Tox. 3 Oral - Eye Irrit. 2 - Skin Irrit. 2 - STOT SE 3
Target Organs
Respiratory system
Supplementary Hazards
Storage Class Code
6.1D - Non-combustible acute toxic Cat.3 / toxic hazardous materials or hazardous materials causing chronic effects
WGK
WGK 2
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Personal Protective Equipment
Certificates of Analysis (COA)
Search for Certificates of Analysis (COA) by entering the products Lot/Batch Number. Lot and Batch Numbers can be found on a product’s label following the words ‘Lot’ or ‘Batch’.
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Articles
Professor Gogotsi and Dr. Shuck introduce MXenes: a promising family of two-dimensional materials with a unique combination of high conductivity, hydrophilicity, and extensive tunability.
Research and development of solid-state lithium fast-ion conductors is crucial because they can be potentially used as solid electrolytes in all-solid-state batteries, which may solve the safety and energy-density related issues of conventional lithium-ion batteries that use liquid (farmable organic) electrolytes.
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service