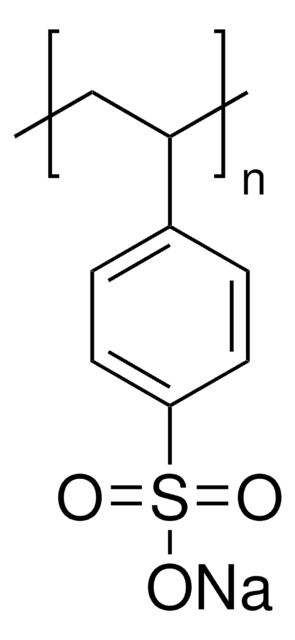
663492
Nafion™ perfluorinated resin solution
20 wt. % in lower aliphatic alcohols and water, contains 34% water
Synonym(s):
D-2020
About This Item
Recommended Products
contains
34% water
greener alternative product characteristics
Design for Energy Efficiency
Learn more about the Principles of Green Chemistry.
sustainability
Greener Alternative Product
concentration
20 wt. % in lower aliphatic alcohols and water
density
1.02 g/mL at 25 °C
greener alternative category
InChI
1S/C7HF13O5S.C2F4/c8-1(9)2(10)24-5(15,16)3(11,4(12,13)14)25-6(17,18)7(19,20)26(21,22)23;3-1(4)2(5)6/h(H,21,22,23);
InChI key
FOYUGSIADQEOEK-UHFFFAOYSA-N
Looking for similar products? Visit Product Comparison Guide
General description
Application
Legal Information
related product
Signal Word
Danger
Hazard Statements
Precautionary Statements
Hazard Classifications
Eye Dam. 1 - Flam. Liq. 2 - Skin Irrit. 2 - STOT SE 3
Target Organs
Central nervous system
Storage Class Code
3 - Flammable liquids
WGK
WGK 2
Flash Point(F)
55.4 °F
Flash Point(C)
13 °C
Certificates of Analysis (COA)
Search for Certificates of Analysis (COA) by entering the products Lot/Batch Number. Lot and Batch Numbers can be found on a product’s label following the words ‘Lot’ or ‘Batch’.
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Articles
Fuel cells have the potential to reduce the nation’s energy use through increased energy conversion efficiency and dependence on imported petroleum by the use of hydrogen from renewable resources.
Proton exchange membrane (PEM) fuel cells operate at relatively low temperatures and are composed of two electrodes and a conductive elecrolyte.
Advances in the electrochemical conversion of water to and from hydrogen and oxygen have principally been achieved through the development of new materials and by understanding the mechanisms of the degradation of proton exchange membrane fuel cells (PEMFC) during operation.
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service