All Photos(2)
About This Item
Empirical Formula (Hill Notation):
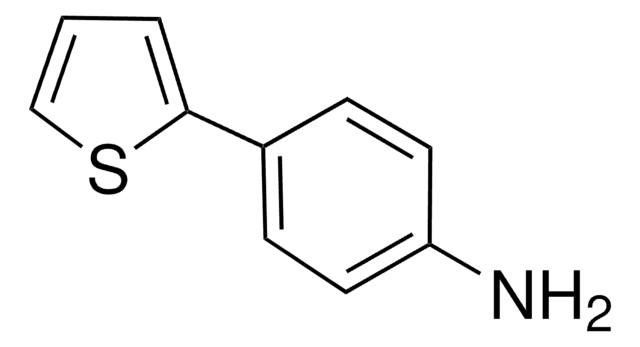
C10H9NS
CAS Number:
Molecular Weight:
175.25
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Assay
97%
form
solid
mp
99-103 °C (lit.)
SMILES string
Nc1ccc(cc1)-c2ccsc2
InChI
1S/C10H9NS/c11-10-3-1-8(2-4-10)9-5-6-12-7-9/h1-7H,11H2
InChI key
GYPDHLDQINBFPY-UHFFFAOYSA-N
Related Categories
Application
4-(Thiophen-3-yl)aniline can be used as a reactant to synthesize:
- Pd(I)–poly[4-(thiophen-3yl)-aniline] composite material by in situ polymerization and composite formation method.
- Schiff base, 2-methoxy-6-[(4-thiophene-3-yl-phenylimino)-methyl]-phenol by condensation reaction with o-vanillin.
- 2-(1H-Imidazol-1-yl)-N-(4-(thiophen-3-yl)phenyl)acetamide by reacting with 2-(1H-imidazol-1-yl)acetic acid using HATU as amide coupling agent.
Signal Word
Danger
Hazard Statements
Precautionary Statements
Hazard Classifications
Acute Tox. 3 Oral - Eye Irrit. 2 - Skin Irrit. 2 - STOT SE 3
Target Organs
Respiratory system
Storage Class Code
6.1C - Combustible acute toxic Cat.3 / toxic compounds or compounds which causing chronic effects
WGK
WGK 3
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Soo Yei Ho et al.
Journal of medicinal chemistry, 60(15), 6678-6692 (2017-07-04)
Porcupine is an O-acyltransferase that regulates Wnt secretion. Inhibiting porcupine may block the Wnt pathway which is often dysregulated in various cancers. Consequently porcupine inhibitors are thought to be promising oncology therapeutics. A high throughput screen against porcupine revealed several
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service