638080
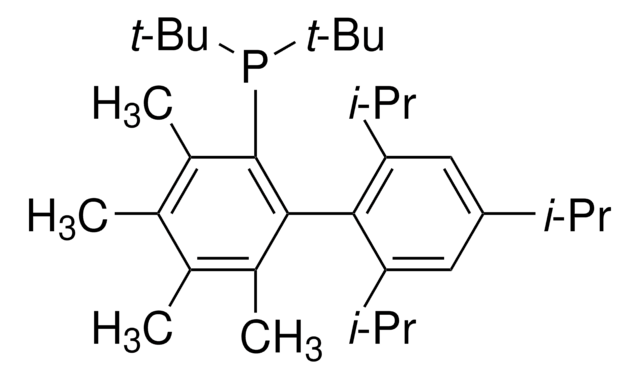
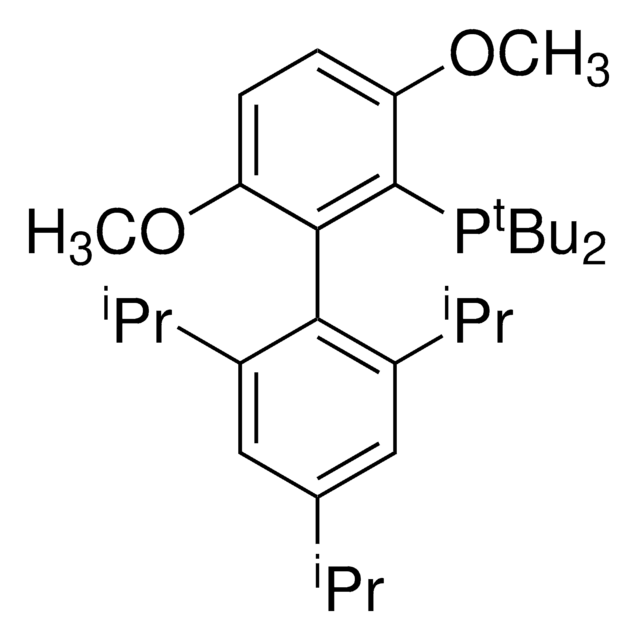
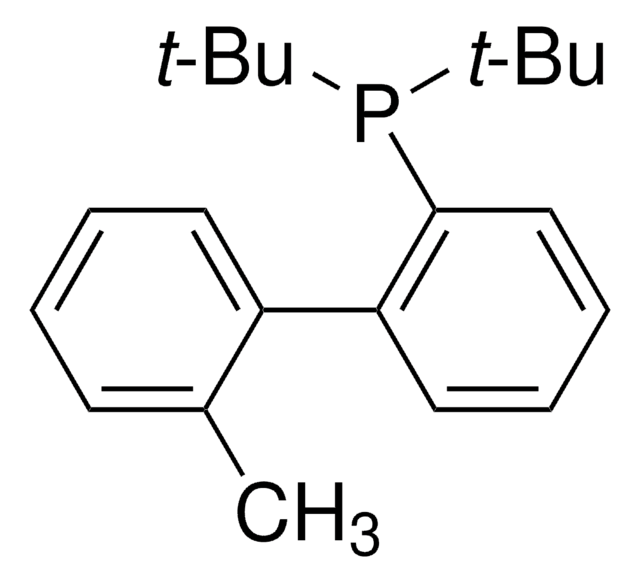
tBuXPhos
98%
Synonym(s):
tBuXPhos, 2-Di-tert-butylphosphino-2′,4′,6′-triisopropylbiphenyl, t-Bu XPhos, tert-Butyl XPhos
About This Item
Recommended Products
Quality Level
Assay
98%
form
solid
reaction suitability
reaction type: Cross Couplings
reagent type: ligand
reaction type: Arylations
reagent type: ligand
reaction type: Buchwald-Hartwig Cross Coupling Reaction
reagent type: ligand
reaction type: Carboxylations
reagent type: ligand
reaction type: Decarboxylations
greener alternative product score
old score: 12
new score: 1
Find out more about DOZN™ Scoring
greener alternative product characteristics
Atom Economy
Design for Energy Efficiency
Use of Renewable Feedstocks
Learn more about the Principles of Green Chemistry.
sustainability
Greener Alternative Product
mp
148-151 °C (lit.)
functional group
phosphine
greener alternative category
SMILES string
CC(P(C(C=CC=C1)=C1C(C(C(C)C)=CC(C(C)C)=C2)=C2C(C)C)C(C)(C)C)(C)C
InChI
1S/C29H45P/c1-19(2)22-17-24(20(3)4)27(25(18-22)21(5)6)23-15-13-14-16-26(23)30(28(7,8)9)29(10,11)12/h13-21H,1-12H3
InChI key
SACNIGZYDTUHKB-UHFFFAOYSA-N
Looking for similar products? Visit Product Comparison Guide
General description
Learn more about Buchwald Phosphine Ligands
Application
It can be used in the following reactions:
- Palladium-catalyzed Tsuji-Trost substitution and cross-coupling of benzylic fluorides.
- Palladium-catalyzed C-N cross-coupling of sulfinamides and aryl halides.
- Palladium-catalyzed rapid methoxylation and deuteriomethoxylation of bromo-chalcones.
Legal Information
related product
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Personal Protective Equipment
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Articles
Buchwald Phosphine Ligands
Buchwald phosphine ligands for C-C, C-N, and C-O bond formation.
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service