All Photos(2)
About This Item
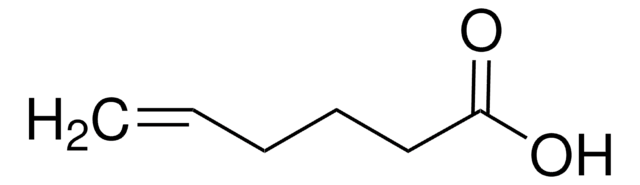
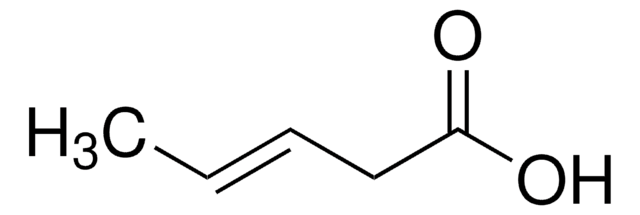
Linear Formula:
CH2=CHCH(CH3)CH2CO2H
CAS Number:
Molecular Weight:
114.14
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Assay
97%
refractive index
n20/D 1.429 (lit.)
bp
75-76 °C/4 mmHg (lit.)
density
0.94 g/mL at 25 °C (lit.)
functional group
carboxylic acid
SMILES string
CC(CC(O)=O)C=C
InChI
1S/C6H10O2/c1-3-5(2)4-6(7)8/h3,5H,1,4H2,2H3,(H,7,8)
InChI key
QNPZXLANENFTFK-UHFFFAOYSA-N
Related Categories
General description
3-Methyl-4-pentenoic acid can be synthesized from crotyl acetate via Claisen rearrangement.
Application
3-Methyl-4-pentenoic acid may be used to synthesize:
- trans- and cis-5-phenylseleno-3-methyl-4-pentanolides
- trans- and cis-5-iodo-3-methyl-4-pentanolides
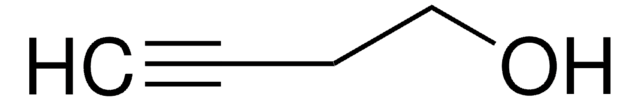
- 3-methyl-4-pentene-1-ol
Signal Word
Danger
Hazard Statements
Precautionary Statements
Hazard Classifications
Eye Dam. 1 - Skin Corr. 1B
Storage Class Code
8A - Combustible corrosive hazardous materials
WGK
WGK 3
Flash Point(F)
199.9 °F - closed cup
Flash Point(C)
93.3 °C - closed cup
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
The ester enolate Claisen rearrangement. Stereochemical control through stereoselective enolate formation
Ireland ER, et al.
Journal of the American Chemical Society, 98(10), 2868-2877 (1976)
Understanding the effect of allylic methyls in olefin cross-metathesis.
Courchay FC, et al.
Journal of Organometallic Chemistry, 691(4), 585-594 (2006)
1, 2-Diferrocenylethane from an Unusual Reaction
Jr R.et al.
Journal of the American Chemical Society, 81(12), 3162-3163 (1959)
Stereoselective selenolactonization by superelectrophilic benzeneselenenyl triflate.
Murata S and Suzuki T.
Chemistry Letters (Jpn), 5, 849-852 (1987)
Back TG.
Organosilicon Chemistry, 40-40 (1999)
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service