535087
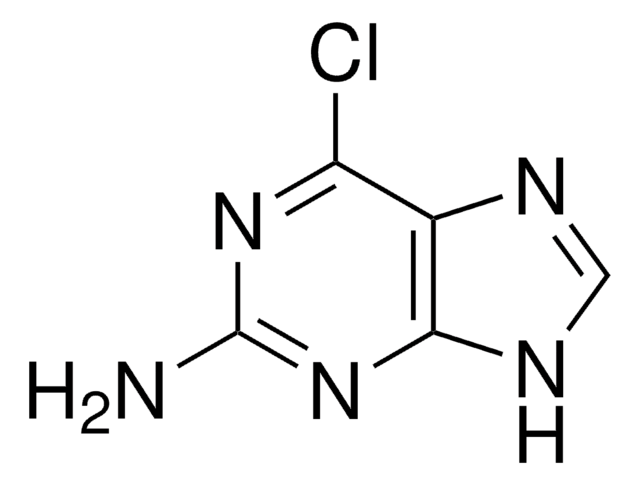
2-Fluoroadenine
96%
Synonym(s):
2-Fluoro-7(9)H-purin-6-ylamine
Sign Into View Organizational & Contract Pricing
All Photos(1)
About This Item
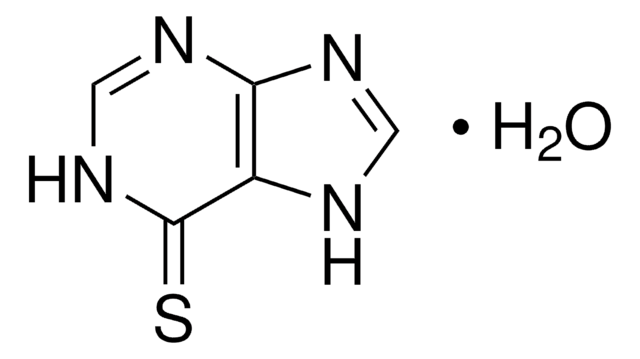
Empirical Formula (Hill Notation):
C5H4FN5
CAS Number:
Molecular Weight:
153.12
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Quality Level
Assay
96%
mp
>350 °C (lit.)
functional group
fluoro
SMILES string
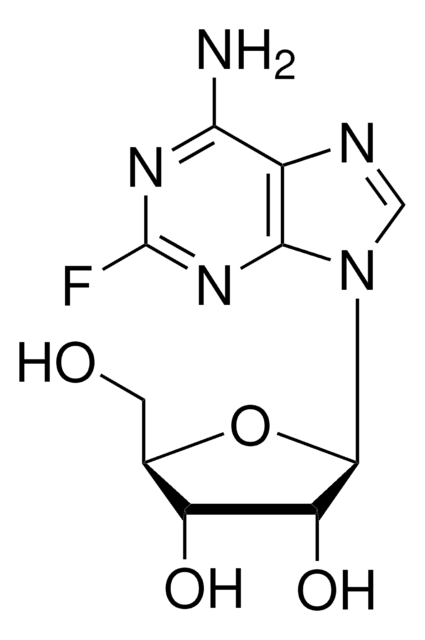
Nc1[nH]c(F)nc2ncnc12
InChI
1S/C5H4FN5/c6-5-10-3(7)2-4(11-5)9-1-8-2/h1H,(H3,7,8,9,10,11)
InChI key
WKMPTBDYDNUJLF-UHFFFAOYSA-N
Signal Word
Warning
Hazard Statements
Precautionary Statements
Hazard Classifications
Eye Irrit. 2 - Skin Irrit. 2 - STOT SE 3
Target Organs
Respiratory system
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Lincoln G Scott et al.
Journal of the American Chemical Society, 126(38), 11776-11777 (2004-09-24)
The production of isotopically labeled RNA remains critical to current NMR structural studies. One approach to obtain simple NMR spectra is to label with a nucleus that is not naturally occurring in RNA. Fluorine-19 can serve as a sensitive site-specific
Yukio Kitade et al.
Nucleic acids research. Supplement (2001), (3)(3), 5-6 (2003-09-27)
Carbocyclic and acyclic nucleosides possessing 2-fluoroadenine, such as 2-fluoronoraristeromycin (6) and 2-fluoro-9-[(2S,3R)-2,3,4-trihydroxy-butyl-1-yl]adenine (8), were synthesized and their inhibitory activities against human and Plasmodium falciparum recombinant SAH hydrolase were investigated.
P Huang et al.
Biochemical pharmacology, 36(18), 2945-2950 (1987-09-15)
2-Fluoroadenine (F-Ade) is a metabolite of 9-beta-D-arabinofuranosyl-2-fluoroadenine (F-ara-A) that may be involved in the development of toxic side effects from this anticancer drug. The liberation of F-Ade from F-ara-A has been examined in different biological systems. Extracts of Escherichia coli
D Voeks et al.
Gene therapy, 9(12), 759-768 (2002-06-01)
A gene-directed enzyme pro-drug therapy (GDEPT) based on purine nucleoside phosphorylase (PNP), that converts the prodrug, fludarabine to 2-fluoroadenine, has been described, but studies are limited compared with other GDEPTs. We investigated the in vitro and in vivo efficacies of
Song Ye et al.
Nucleosides, nucleotides & nucleic acids, 22(10), 1899-1905 (2003-11-12)
A convenient synthesis of 2'-deoxy-2-fluoroadenosine from commercially available 2-fluoroadenine is described. The coupling reaction of silylated 2-fluoroadenine with phenyl 3,5-bis[O-(t-butyldimethylsilyl)]-2-deoxy-1-thio-D-erythro-pentofuranoside gave the corresponding 2-fluoro-2'-deoxyadenosine derivative (alpha/beta = 1:1) in good yield. The alpha- and beta-anomers were separated by chromatography, and
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service