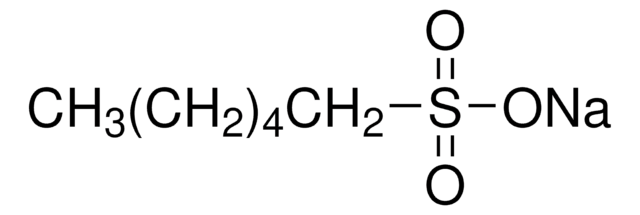52865
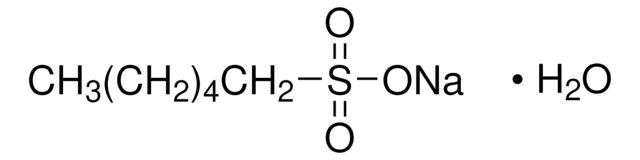
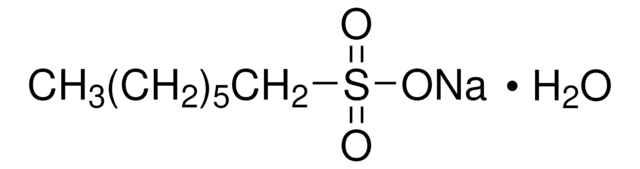
Sodium 1-hexanesulfonate monohydrate
≥98.0% (T)
Synonym(s):
1-Hexanesulfonic acid sodium salt monohydrate
Sign Into View Organizational & Contract Pricing
All Photos(2)
About This Item
Linear Formula:
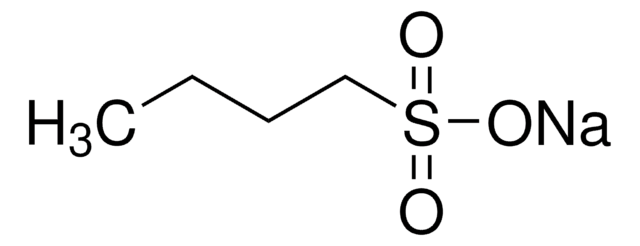
CH3(CH2)4CH2SO3Na · H2O
CAS Number:
Molecular Weight:
206.24
Beilstein:
3727014
EC Number:
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Quality Level
Assay
≥98.0% (T)
form
solid
SMILES string
O.[Na+].CCCCCCS([O-])(=O)=O
InChI
1S/C6H14O3S.Na.H2O/c1-2-3-4-5-6-10(7,8)9;;/h2-6H2,1H3,(H,7,8,9);;1H2/q;+1;/p-1
InChI key
CLCGFJYKZGFGSQ-UHFFFAOYSA-M
Looking for similar products? Visit Product Comparison Guide
Related Categories
Application
Sodium 1-hexanesulfonate monohydrate can be used as a catalyst to synthesize:
It can also be used as a reactant to prepare polynuclear oxidobismuth sulfonates by reacting with sodium 2-methyl-2-propensulfonate and tetra-μ3-hydroxyhexakis(nitrato-κO)tetra-μ3-oxohexabismuth in DMSO.
- Amidoalkyl naphthols by three-component reaction of β-naphthol, aldehydes, and urea.
- Aminophosphonates by the reaction of aldehydes/ketones with amines.
It can also be used as a reactant to prepare polynuclear oxidobismuth sulfonates by reacting with sodium 2-methyl-2-propensulfonate and tetra-μ3-hydroxyhexakis(nitrato-κO)tetra-μ3-oxohexabismuth in DMSO.
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Kirti S Niralwad et al.
Ultrasonics sonochemistry, 17(5), 760-763 (2010-03-17)
1-Hexanesulphonic acid sodium salt was found to be an efficient catalyst for the green synthesis of alpha-aminophosphonates by the coupling of aldehydes/ketone, an amine and triethyl phosphite under ultrasound irradiation at ambient temperature for appropriate time to furnish the desired
Philip Zakaria et al.
Electrophoresis, 23(17), 2821-2832 (2002-09-11)
The separation of a series of aromatic carboxylic acids, sulfonates and opiates using electrokinetic chromatography employing a mixture of the soluble cationic polymer poly(diallydimethylammonium chloride) (PDDAC) and the amphiphilic anion hexanesulfonate as pseudostationary phases is described. In this system, the
A R Calhoun et al.
Journal of colloid and interface science, 309(2), 505-510 (2007-03-03)
Measurements have been made to determine the solubility of ethane, C2H6, in aqueous solutions of four different surfactants of the linear alkanesulfonate class at 25 degrees C. The surfactants, sodium 1-pentanesulfonate, sodium 1-hexanesulfonate, sodium 1-heptanesulfonate, and sodium 1-octanesulfonate, all share
Ana L Costa et al.
Chemistry (Weinheim an der Bergstrasse, Germany), 21(34), 12069-12078 (2015-07-29)
Zn-Al layered double hydroxides (LDHs) containing solely indigo carmine (IC) or 1-hexanesulfonate (HS) anions, or a mixture of the two with different HS/IC molar ratios, were prepared by the direct synthesis method and characterized by various techniques. Hydrotalcite-type phases were
Mixtures of catanionic surfactants can be superspreaders: Comparison with trisiloxane superspreader.
N M Kovalchuk et al.
Journal of colloid and interface science, 459, 250-256 (2015-08-25)
Mixed solutions of cationic and anionic surfactants show considerable synergism in their wetting behaviour, but their spreading is affected considerably by the phase separation processes. The valuable information about wetting properties of synergetic mixtures can be obtained by using mixtures
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service