523054
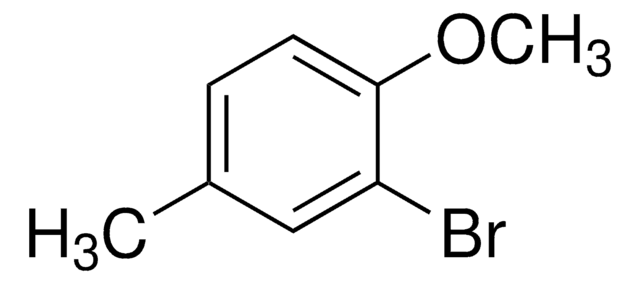
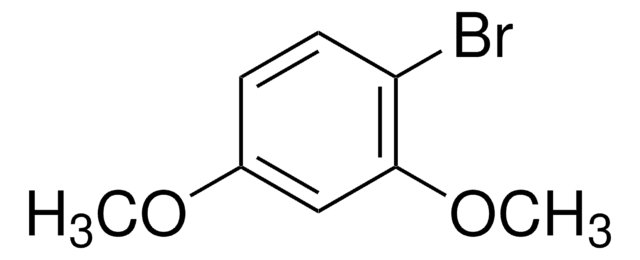
4-Bromo-2-methylanisole
98%
Synonym(s):
5-Bromo-2-methoxytoluene
Sign Into View Organizational & Contract Pricing
All Photos(1)
About This Item
Linear Formula:
BrC6H3(CH3)OCH3
CAS Number:
Molecular Weight:
201.06
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Quality Level
Assay
98%
form
solid
mp
66-69 °C (lit.)
functional group
bromo
SMILES string
COc1ccc(Br)cc1C
InChI
1S/C8H9BrO/c1-6-5-7(9)3-4-8(6)10-2/h3-5H,1-2H3
InChI key
UDLRGQOHGYWLCS-UHFFFAOYSA-N
General description
4-Bromo-2-methylanisole can be prepared by the bromination of o-methylanisole (2-methylanisole). It can also be prepared via reaction between 1-butyl-3-methylimidazolium tribromide [(Bmim)Br3] and an activated aromatic compound. It participates as a substrate for the a-arylation of sulphonamide in a study.
Application
4-Bromo-2-methylanisole may be used in the synthesis of 4-isopropyl-2-methylphenol and 4-methoxy-3-methylphenol.
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Certificates of Analysis (COA)
Lot/Batch Number
Don't see the Right Version?
If you require a particular version, you can look up a specific certificate by the Lot or Batch number.
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
(Bmim) Br3 as a New Reagent for Regioselective Monobromination of Phenols and Several Activated Aromatics under Solvent-free Conditions.
Le ZG, et al.
Chin. J. Chem., 23(11), 1537-1540 (2005)
James R Vyvyan et al.
The Journal of organic chemistry, 69(7), 2461-2468 (2004-03-31)
Aromatic bisabolene derivatives were prepared by two methods involving cross-coupling of organozinc reagents. The first synthesis of (+/-)-glandulone A (10), as well as syntheses of (+/-)-curcuhydroquinone (8) and (+/-)-curcuquinone (9), were accomplished via coupling of a secondary alkyl zinc reagent
Total synthesis of (?)-heliannuol D, an allelochemical from Helianthus annuus.
Vyvyan JR and Looper RE.
Tetrahedron Letters, 41(8), 1151-1154 (2000)
The isopropyl cresols.
Carpenter MS and Easter WM.
The Journal of Organic Chemistry, 20(4), 401-411 (1955)
George Majetich et al.
The Journal of organic chemistry, 62(13), 4321-4326 (1997-06-27)
It has been shown that bromodimethylsulfonium bromide, generated in situ by treating dimethyl sulfoxide with aqueous hydrobromic acid, is a milder and more selective reagent for electrophilic aromatic bromination than elemental bromine.
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service