All Photos(1)
About This Item
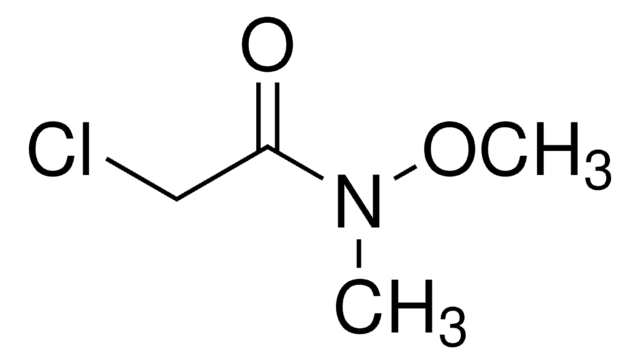
Linear Formula:
CH3CON(OCH3)CH3
CAS Number:
Molecular Weight:
103.12
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Quality Level
Assay
98%
refractive index
n20/D 1.426 (lit.)
bp
152 °C (lit.)
density
0.97 g/mL at 25 °C (lit.)
functional group
amine
SMILES string
CON(C)C(C)=O
InChI
1S/C4H9NO2/c1-4(6)5(2)7-3/h1-3H3
InChI key
OYVXVLSZQHSNDK-UHFFFAOYSA-N
Related Categories
General description
Simple Weinreb amide used in the synthesis of marine natural products myriaporone and usneoidone as a ketone synthon.
Signal Word
Warning
Hazard Statements
Precautionary Statements
Hazard Classifications
Flam. Liq. 3
Storage Class Code
3 - Flammable liquids
WGK
WGK 3
Flash Point(F)
120.2 °F - closed cup
Flash Point(C)
49 °C - closed cup
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Total synthesis of natural myriaporones.
Marta Pérez et al.
Angewandte Chemie (International ed. in English), 43(13), 1724-1727 (2004-03-24)
Asymmetric synthesis of heterocycles using sulfinimines (N-sulfinyl imines).
Davis FA, et al.
ARKIVOC (Gainesville, FL, United States), 7, 120-128 (2006)
European Journal of Organic Chemistry, 9, 1911-1922 (2004)
Acetylations of strongly basic and nucleophilic enolate anions with N-methoxy-N-methylacetamide.
Oster TA and Harris TM.
Tetrahedron Letters, 24(18), 1851-1854 (1983)
Xue Zhi Zhao et al.
Bioorganic & medicinal chemistry, 14(23), 7816-7825 (2006-08-16)
The diketo acid (DKA) class of HIV-1 integrase inhibitors are thought to function by chelating divalent metal ions within the enzyme catalytic center. However, differences in mutations conferring resistance among sub-families of DKA inhibitors suggest that multiple binding orientations may
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service