All Photos(1)
About This Item
Empirical Formula (Hill Notation):
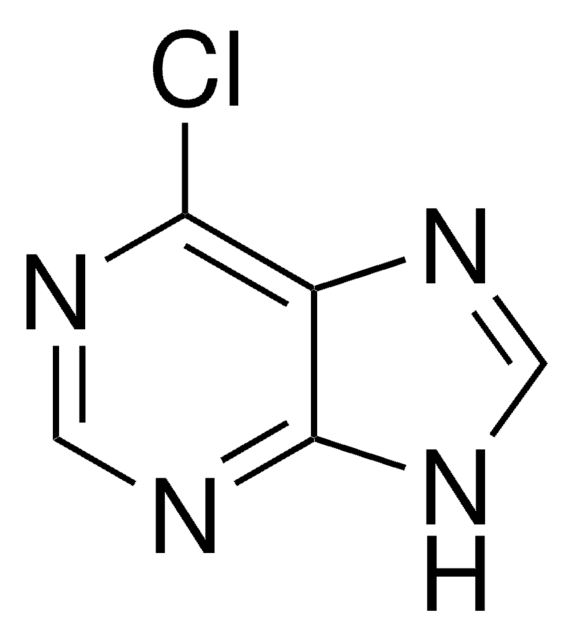
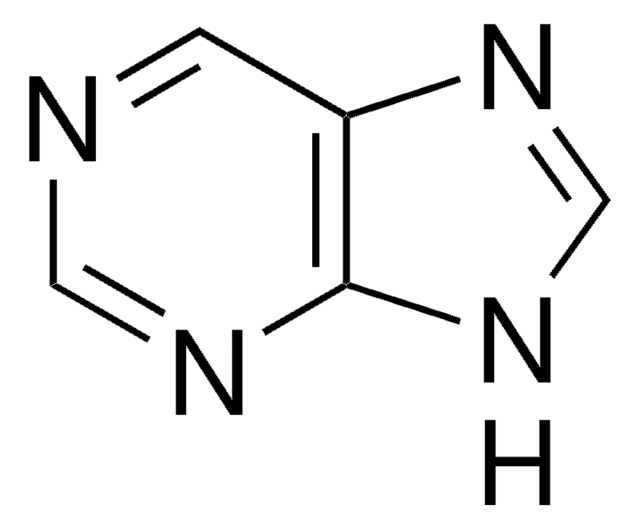
C5H3ClN4
CAS Number:
Molecular Weight:
154.56
Beilstein:
5774
EC Number:
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Quality Level
Assay
99%
mp
>300 °C (dec.) (lit.)
functional group
chloro
SMILES string
Clc1ncnc2[nH]cnc12
InChI
1S/C5H3ClN4/c6-4-3-5(9-1-7-3)10-2-8-4/h1-2H,(H,7,8,9,10)
InChI key
ZKBQDFAWXLTYKS-UHFFFAOYSA-N
Looking for similar products? Visit Product Comparison Guide
General description
6-Chloropurine (6-CIPH), a 6-substituted purine derivative, is an antileukemic drug. It can be prepared by the chlorination of hypoxanthine with phosphorus oxychloride in the presence of dimethylaniline. The NMR-based conformational analysis of the products formed during the reaction of 6-CIPH with 3,4-di-O-acetyl-D-xylal and 3,4-di-O-acetyl-L-arabinal have been reported. 6-CIPH can undergo palladium-catalyzed cross coupling with organostannanes at 6-position to form the corresponding arylated or alkylated products.
Application
6-Chloropurine may be used:
- To prepare purine via catalytic dehydrogenation.
- To prepare 9-alkylated adenines via Mitsunobu reaction with various alcohols.
- As a starting material to synthesize dihydroisoxazole 6-chloropurine.
Signal Word
Warning
Hazard Statements
Hazard Classifications
Acute Tox. 4 Oral
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Synthesis and anti-HIV activity of dihydroisoxazole 6-chloropurine and adenine.
Xiang Y, et al.
Bioorganic & Medicinal Chemistry Letters, 6(9), 1051-1054 (1996)
Synthesis of Nucleosides and Related Compounds. Part XXV. The Alkylation of 6-Chloropurine with Alcohols by Mitsunobu Reaction.
Toyota A, et al.
Chemical & Pharmaceutical Bulletin, 40(4), 1039-1041 (1992)
6-Chloropurines and organostannanes in palladium catalyzed cross coupling reactions.
Gundersen LL.
Tetrahedron Letters, 35(19), 3155-3158 (1994)
The Synthesis and Properties of 6-Chloropurine and Purine1.
Bendich A, et al.
Journal of the American Chemical Society, 76(23), 6073-6077 (1954)
REACTIONS OF RIBONUCLEOTIDE DERIVATIVES OF PURINE ANALOGUES AT THE CATALYTIC SITE OF INOSINE 5'-PHOSPHATE DEHYDROGENASE.
A HAMPTON
The Journal of biological chemistry, 238, 3068-3074 (1963-09-01)
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service