438561
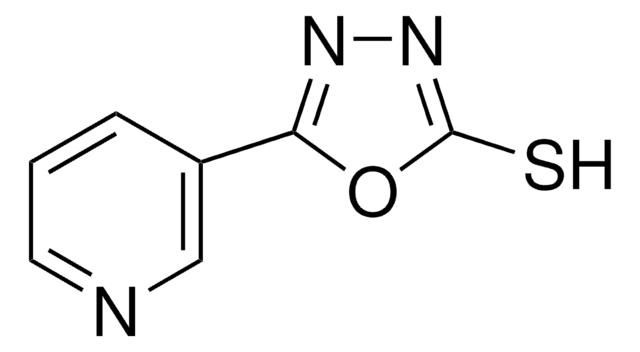
5-(4-Pyridyl)-1,3,4-oxadiazole-2-thiol
97%
Synonym(s):
5-(4-Pyridinyl)-1,3,4-oxadiazole-2-thiol, 5-(4-Pyridyl)-1,3,4-oxadiazole-2(3H )-thione, 5-(4-Pyridyl)-1,3,4-oxadiazole-2-thione, 5-Pyridin-4-yl-[1,3,4]oxadiazol-2-thiol
Sign Into View Organizational & Contract Pricing
All Photos(1)
About This Item
Empirical Formula (Hill Notation):
C7H5N3OS
CAS Number:
Molecular Weight:
179.20
MDL number:
UNSPSC Code:
12352103
PubChem Substance ID:
NACRES:
NA.23
Recommended Products
Assay
97%
mp
270-275 °C (lit.)
SMILES string
Sc1nnc(o1)-c2ccncc2
InChI
1S/C7H5N3OS/c12-7-10-9-6(11-7)5-1-3-8-4-2-5/h1-4H,(H,10,12)
InChI key
TXCXZVFDWQYTIC-UHFFFAOYSA-N
General description
5-(4-Pyridyl)-1,3,4-oxadiazole-2-thiol is a pyridyl oxadiazole based building block that has a 1,3,4-oxadiazole as the central ring, which is attached with pyridyl and thiol as pendant groups. It has a luminescence property that makes it useful in biological applications. It can also be used as a bridging ligand for the formation of novel polymeric coordination systems.
Application
5-(4-Pyridyl)-1,3,4-oxadiazole-2-thiol can be used in the formation of metal organic frameworks (MOFs), which include supramolecules and co-ordination polymers that find potential applications in ion exchange, catalysis, and storage of gases.
Signal Word
Warning
Hazard Statements
Precautionary Statements
Hazard Classifications
Eye Irrit. 2 - Skin Irrit. 2 - STOT SE 3
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Novel HgII and MnII supramolecular complexes with a versatile building block 5-(4-pyridyl)-1, 3, 4-oxadiazole-2-thiolate involving in situ ligand formation
Zhang S, et al.
Inorganic Chemistry Communications, 12(10), 1038-1041 (2009)
Hg (II) complexes of 4-phenyl-5-(3-pyridyl)-1, 2, 4-triazole-3-thione and 5-(4-pyridyl)-1, 3, 4-oxadiazole-2-thione and a Ni (II) complex of 5-(thiophen-2-yl)-1, 3, 4-oxadiazole-2-thione: Synthesis and X-ray structural studies
Bharti A, et al.
Polyhedron, 50(1), 582-591 (2013)
Palraj Kalimuthu et al.
Chemistry (Weinheim an der Bergstrasse, Germany), 24(30), 7710-7717 (2018-03-25)
The electrochemically driven catalysis of the complex molybdoenzyme steroid C25 dehydrogenase (S25DH) from the β-Proteobacterium Sterolibacterium denitrificans is reported. S25DH catalyses the oxygen-independent regioselective hydroxylation of the tertiary C25 atom of sterols and also their derivatives. Cholest-4-en-3-one is a native
Homan Kang et al.
Scientific reports, 5, 10144-10144 (2015-05-29)
Recently, preparation and screening of compound libraries remain one of the most challenging tasks in drug discovery, biomarker detection, and biomolecular profiling processes. So far, several distinct encoding/decoding methods such as chemical encoding, graphical encoding, and optical encoding have been
Global Trade Item Number
| SKU | GTIN |
|---|---|
| 438561-1G |
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service