430595
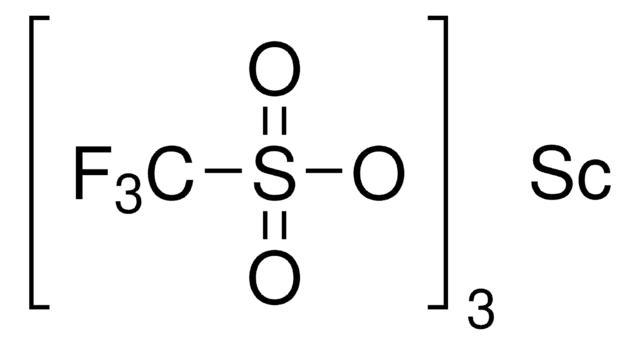
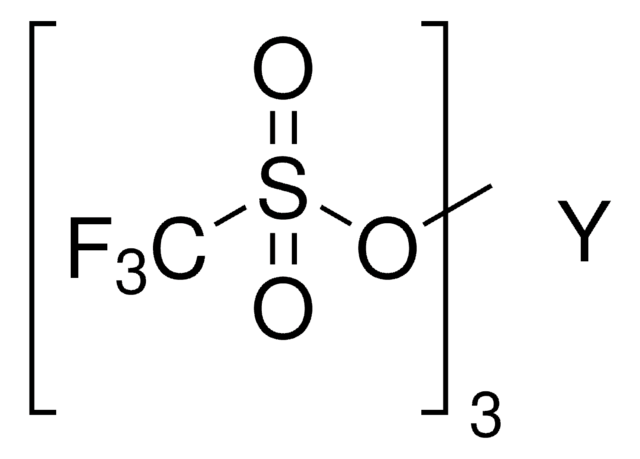
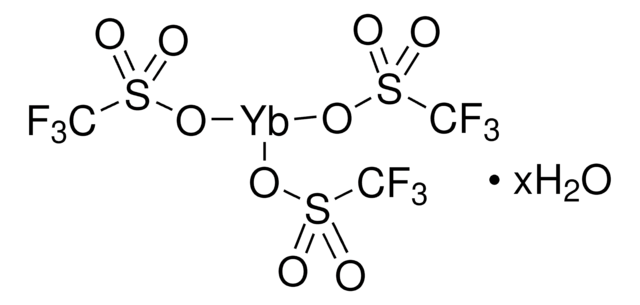
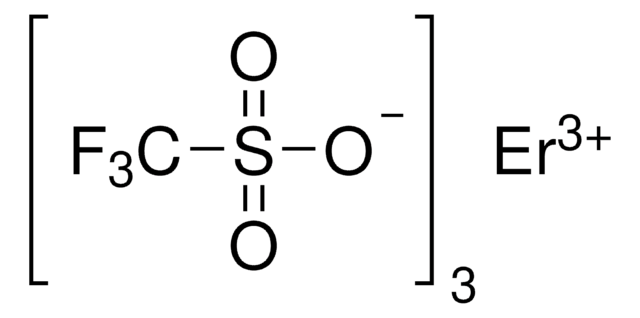
Ytterbium(III) trifluoromethanesulfonate
99.99%
Synonym(s):
Yb(OTf)3, Trifluoromethanesulfonic acid ytterbium(III) salt, Yb(TFA)3, Ytterbium(III) triflate
Sign Into View Organizational & Contract Pricing
All Photos(1)
About This Item
Linear Formula:
(CF3SO3)3Yb
CAS Number:
Molecular Weight:
620.25
MDL number:
UNSPSC Code:
12161600
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Assay
99.99%
reaction suitability
core: ytterbium
reagent type: catalyst
SMILES string
FC(F)(F)S(=O)(=O)O[Yb](OS(=O)(=O)C(F)(F)F)OS(=O)(=O)C(F)(F)F
InChI
1S/3CHF3O3S.Yb/c3*2-1(3,4)8(5,6)7;/h3*(H,5,6,7);/q;;;+3/p-3
InChI key
AHZJKOKFZJYCLG-UHFFFAOYSA-K
Related Categories
Application
A water-tolerant Lewis acid catalyst recently used in one-pot syntheses of β-lactams, and in stereocontrolled radical and nucleophilic addition reactions. Catalyzes the synthesis of deoxypenostatin A in a novel, stereoselective, intramolecular Diels-Alder reaction.
Lewis acid catalyst used with a silylated chinchona alkaloid in an asymmetric synthesis of substituted β-lactams from an acid chloride and aryl imine. Also used to catalyze an aldehyde-ene reaction in a synthesis of the guaiane skeleton.
An important Lewis acid catalyst for a variety of synthetic reactions, including allylborations.
Lewis acid catalyst used with a silylated chinchona alkaloid in an asymmetric synthesis of substituted β-lactams from an acid chloride and aryl imine. Also used to catalyze an aldehyde-ene reaction in a synthesis of the guaiane skeleton.
An important Lewis acid catalyst for a variety of synthetic reactions, including allylborations.
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Yb(OTf)(3)-Catalyzed One-Pot Synthesis of beta-Lactams from Silyl Ketene Thioacetals by a Two- or a Three-Component Reaction.
Rita Annunziata et al.
The Journal of organic chemistry, 61(23), 8293-8296 (1996-11-15)
Aldrichimica Acta, 28, 77-77 (1995)
Tetrahedron Asymmetry, 7, 1457-1457 (1996)
Kobayashi, S.
Transition Metals for Organic Synthesis, 1, 285-285 (1998)
Jili Li et al.
Organic & biomolecular chemistry, 16(43), 8115-8129 (2018-10-20)
CO-releasing molecules (CORMs) containing cobalt have many bioactivities, but most of them do not dissolve in water and have no selectivity to tissue and organs. On the basis of the specific recognition of galactose or sialic acid by a receptor
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service