428507
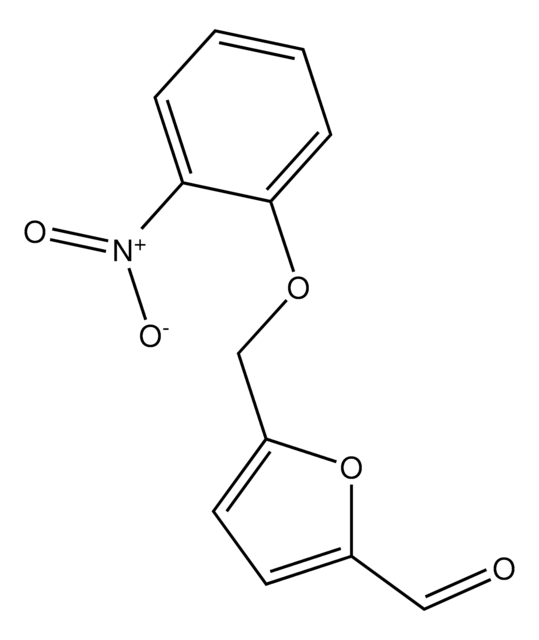
5-(2-Nitrophenyl)furfural
99%
Synonym(s):
5-(2-Nitrophenyl)-2-furancarboxaldehyde
Sign Into View Organizational & Contract Pricing
All Photos(1)
About This Item
Empirical Formula (Hill Notation):
C11H7NO4
CAS Number:
Molecular Weight:
217.18
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Quality Level
Assay
99%
form
solid
bp
105-107 °C/30 mmHg (lit.)
mp
94-97 °C (lit.)
functional group
aldehyde
nitro
SMILES string
[H]C(=O)c1ccc(o1)-c2ccccc2[N+]([O-])=O
InChI
1S/C11H7NO4/c13-7-8-5-6-11(16-8)9-3-1-2-4-10(9)12(14)15/h1-7H
InChI key
QBYRUURYXPVDAK-UHFFFAOYSA-N
General description
5-(2-Nitrophenyl)furfural is a furfural derivative.
Application
5-(2-Nitrophenyl)furfural (5-(2-nitrophenyl)-2-furfuraldehyde) was used in the synthesis of (5-(2-nitrophenyl)furfuran-2-yl)methanol.
It may be used in the synthesis of 2-{[5-(2-nitrophenyl)furan-2-yl]methyleneamino}benzoic acid (HOBZ) by reacting with 2-aminobenzoic acid.
It may be used in the synthesis of 2-{[5-(2-nitrophenyl)furan-2-yl]methyleneamino}benzoic acid (HOBZ) by reacting with 2-aminobenzoic acid.
Signal Word
Warning
Hazard Statements
Precautionary Statements
Hazard Classifications
Eye Irrit. 2 - Skin Irrit. 2 - STOT SE 3
Target Organs
Respiratory system
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
New di-and triorganotin (IV) carboxylates derived from a Schiff base: synthesis, characterization and in vitro antimicrobial activities.
Dias LC, et al.
Applied Organometallic Chemistry, 29(5), 305-313 (2015)
Ruthenium (II)-Catalyzed Transfer Hydrogenation of Aromatic and Heteroaromatic Aldehydes in Air.
Bhosale SS and Singh KS.
Synthetic Communications, 45(12), 1-10 (2015)
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service