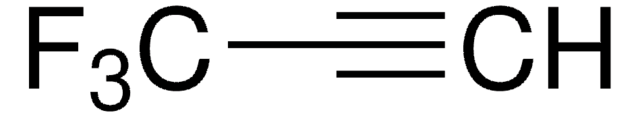All Photos(1)
About This Item
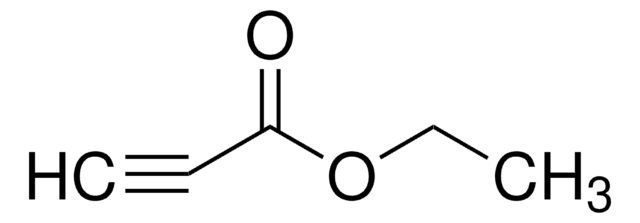
Linear Formula:
CF3C≡CCO2C2H5
CAS Number:
Molecular Weight:
166.10
Beilstein:
3539414
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Quality Level
Assay
97%
form
liquid
refractive index
n20/D 1.350 (lit.)
bp
96-98 °C (lit.)
density
1.162 g/mL at 25 °C (lit.)
functional group
ester
fluoro
SMILES string
CCOC(=O)C#CC(F)(F)F
InChI
1S/C6H5F3O2/c1-2-11-5(10)3-4-6(7,8)9/h2H2,1H3
InChI key
SFDRHPQGYUYYNX-UHFFFAOYSA-N
Looking for similar products? Visit Product Comparison Guide
General description
Ethyl 4,4,4-trifluoro-2-butynoate is an unsymmetrical internal alkyne.
Application
Ethyl 4,4,4-trifluoro-2-butynoate has been used to investigate the regioselectivity of the insertion reaction with cyclometalated iridium and rhodium complexes.
It may be used in the synthesis of the following compounds :
It may be used in the synthesis of the following compounds :
- (2S,4aR,6aR,7R,9S,10aS,10bR)-methyl 9-acetoxy-2-(5-(ethoxycarbonyl)-6-(trifluoromethyl)-7-oxabicyclo[2.2.1]hepta-2,5-dien-2-yl)-6a,10b-dimethyl-4,10-dioxododecahydro-1H-benzo[f]isochromene-7-carboxylate
- (2S,4aR,6aR,7R,9S,10aS,10bR)-methyl 9-acetoxy-2-(6-(ethoxycarbonyl)-5-(trifluoromethyl)-7-oxabicyclo[2.2.1]hepta-2,5-dien-2-yl)-6a,10b-dimethyl-4,10-dioxododecahydro-1Hbenzo[f]isochromene-7-carboxylate
Signal Word
Danger
Hazard Statements
Precautionary Statements
Hazard Classifications
Eye Irrit. 2 - Flam. Liq. 2 - Skin Irrit. 2 - STOT SE 3
Target Organs
Respiratory system
Storage Class Code
3 - Flammable liquids
WGK
WGK 3
Flash Point(F)
42.8 °F - closed cup
Flash Point(C)
6 °C - closed cup
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Reactivity and Regioselectivity of Insertion of Unsaturated Molecules into M- C (M= Ir, Rh) Bonds of Cyclometalated Complexes.
Li L, et al.
Organometallics, 29(20), 4593-4605 (2010)
Anthony Lozama et al.
Journal of natural products, 74(4), 718-726 (2011-02-23)
As part of our continuing efforts toward more fully understanding the structure-activity relationships of the neoclerodane diterpene salvinorin A, we report the synthesis and biological characterization of unique cycloadducts through [4+2] Diels-Alder cycloaddition. Microwave-assisted methods were developed and successfully employed
Facile access to stereodefined dienoates and cyclopropylenoates containing a trifluoromethyl group.
Wang P-A, et al.
Journal of Fluorine Chemistry, 124(1), 93-97 (2003)
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service