All Photos(1)
About This Item
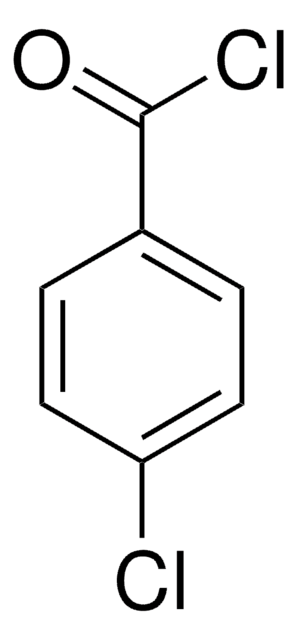
Linear Formula:
ClC6H4COCl
CAS Number:
Molecular Weight:
175.01
Beilstein:
471606
EC Number:
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Assay
95%
form
liquid
refractive index
n20/D 1.578 (lit.)
bp
102-104 °C/11 mmHg (lit.)
mp
11-14 °C (lit.)
density
1.365 g/mL at 20 °C (lit.)
functional group
acyl chloride
chloro
SMILES string
ClC(=O)c1ccc(Cl)cc1
InChI
1S/C7H4Cl2O/c8-6-3-1-5(2-4-6)7(9)10/h1-4H
InChI key
RKIDDEGICSMIJA-UHFFFAOYSA-N
Looking for similar products? Visit Product Comparison Guide
General description
4-Chlorobenzoyl chloride is an acyl chloride. It reacts with 2-amino-2-seleno-5,5-dimethyl-1,3,2-dioxaphosphorinane to yield the respective N-acyl selenophosphoramides.
Application
4-Chlorobenzoyl chloride may be used in the following studies:
- Acylation of benzene using different solid acid catalysts such as dodecatungstophosphoric acid (DTPA), DTPA/K-10 clay, K-10, Amberlite, Amberlyst-15, Indion-130, Filtrol-24 clay and sulfated zirconia.
- Preparation of 1-(4-chlorobenzoyl)-2, 7-dimethoxynaphthalene.
- Preparation of 4-chlorobenzoyl CoA, via reaction with CoA in KHCO3 buffer.
- Preparation of 1-aryloxyacetyl-4-(4-chlorobenzoyl)-semicarbazides.
Signal Word
Danger
Hazard Statements
Precautionary Statements
Hazard Classifications
Aquatic Chronic 3 - Eye Dam. 1 - Skin Corr. 1B
Storage Class Code
8A - Combustible corrosive hazardous materials
WGK
WGK 1
Flash Point(F)
221.0 °F - closed cup
Flash Point(C)
105 °C - closed cup
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Friedel-Crafts acylation using sulfated zirconia catalyst.
Yadav GD and Pujari AA.
Green Chemistry, 1(2), 69-74 (1999)
A Facile Method to 1, 4-Diacyl Semicarbazides: Syntheses of 1-Aryloxyacetyl-4-(4-Chlorobenzoyl)-Thiosemicarbazides and Semicarbazides.
Wang X, et al.
Synthetic Communications, 30(18), 3405-3411 (2000)
Ryosuke Mitsui et al.
Acta crystallographica. Section E, Structure reports online, 64(Pt 7), o1278-o1278 (2008-01-01)
In the title compound, C(19)H(15)ClO(3), the dihedral angle between the naphthalene ring system and the benzene ring is 72.06 (7)°. The 4-chloro-phenyl group and the carbonyl group are almost coplanar. An inter-molecular C-H⋯O hydrogen bond is formed between an H atom
S D Copley et al.
Applied and environmental microbiology, 58(4), 1385-1387 (1992-04-01)
4-Chlorobenzoate degradation in cell extracts of Acinetobacter sp. strain 4-CB1 occurs by initial synthesis of 4-chlorobenzoyl coenzyme A (4-chlorobenzoyl CoA) from 4-chlorobenzoate, CoA, and ATP. 4-Chlorobenzoyl CoA is dehalogenated to 4-hydroxybenzoyl CoA. Following the dehalogenation reaction, 4-hydroxybenzoyl CoA is hydrolyzed
Grzegorz Cholewinski et al.
Organic & biomolecular chemistry, 7(19), 4095-4100 (2009-09-19)
2-Amino-2-seleno-5,5-dimethyl-1,3,2-dioxaphosphorinane reacts with acyl chlorides (4-chlorobenzoyl chloride or pivaloyl chloride) yielding the respective N-acyl selenophosphoramides. These derivatives do not isomerise to the related selenocarbonyl imides. X-ray study of N-(4-chlorobenzoyl)-2-amino-2-seleno-5,5-dimethyl-1,3,2-dioxaphosphorinane indicates that the selenium atom is placed in the equatorial position.
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service