All Photos(2)
About This Item
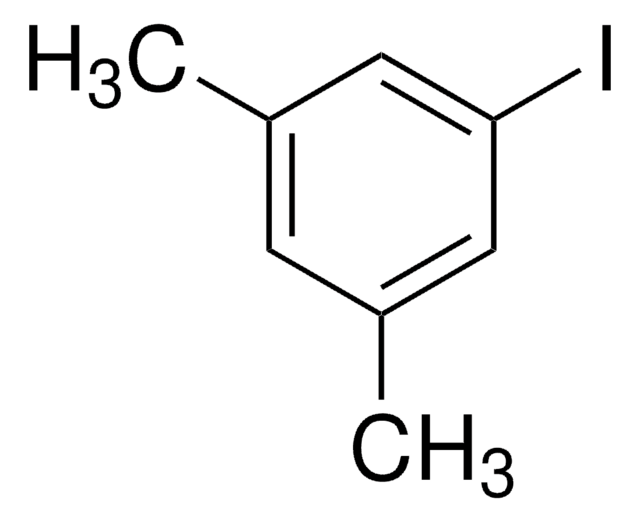
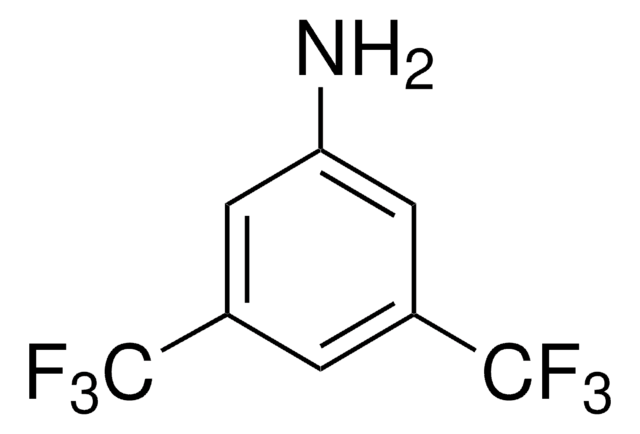
Linear Formula:
(CF3)2C6H3I
CAS Number:
Molecular Weight:
340.00
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Assay
98%
form
liquid
refractive index
n20/D 1.463 (lit.)
bp
59-61 °C/10 mmHg (lit.)
density
1.919 g/mL at 25 °C (lit.)
SMILES string
FC(F)(F)c1cc(I)cc(c1)C(F)(F)F
InChI
1S/C8H3F6I/c9-7(10,11)4-1-5(8(12,13)14)3-6(15)2-4/h1-3H
InChI key
VDPIZIZDKPFXLI-UHFFFAOYSA-N
General description
1-Iodo-3,5-bis(trifluoromethyl)benzene is a halogenated hydrocarbon.
Application
1-Iodo-3,5-bis(trifluoromethyl)benzene may be used in the preparation of [bis(trifluoroacetoxy)iodo]perfluoroalkanes. It may be used to investigate the fragmentation reactions of (E)- and (Z)-2-methylbuten-1-yl(aryl)iodonium triflates (aryl = C6H5-, 4-(CF3)C6H4, 3,5-(CF3)2C6H4-).
Signal Word
Warning
Hazard Statements
Precautionary Statements
Hazard Classifications
Eye Irrit. 2 - Skin Irrit. 2 - STOT SE 3
Target Organs
Respiratory system
Storage Class Code
10 - Combustible liquids
WGK
WGK 3
Flash Point(F)
165.2 °F - closed cup
Flash Point(C)
74 °C - closed cup
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Aleksandra A Zagulyaeva et al.
The Journal of organic chemistry, 75(6), 2119-2122 (2010-02-19)
[Bis(trifluoroacetoxy)iodo]perfluoroalkanes C(n)F(2n+1)I(OCOCF(3))(2) (n = 4, 6, 8, 10, 12) can be conveniently prepared by the oxidation of the corresponding perfluoroalkyl iodides with Oxone in trifluoroacetic acid at room temperature and subsequently converted to the stable [hydroxy(tosyloxy)iodo]perfluoroalkanes, C(n)F(2n+1)I(OH)OTs, by treatment with
Fragmentations of (E)-and (Z)-isomers of 2-methylbuten-1-yl (aryl) iodonium triflates: competing mechanisms for enol triflate formation.
Hinkle RJ.
ARKIVOC (Gainesville, FL, United States), 6, 201-212 (2003)
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service




![[Bis(trifluoroacetoxy)iodo]benzene 97%](/deepweb/assets/sigmaaldrich/product/structures/238/293/71fcde9a-4afb-4cf5-9c22-8d8d68bf1ba4/640/71fcde9a-4afb-4cf5-9c22-8d8d68bf1ba4.png)