All Photos(1)
About This Item
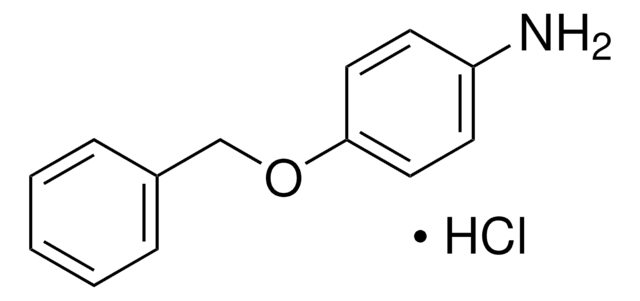
Empirical Formula (Hill Notation):
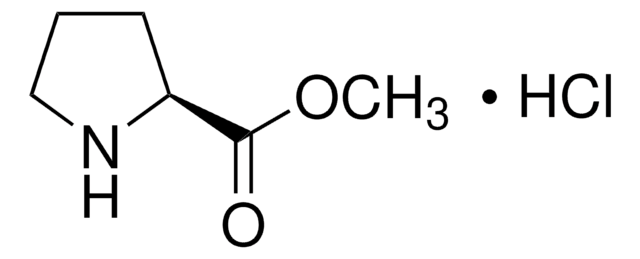
C12H15NO2 · HCl
CAS Number:
Molecular Weight:
241.71
Beilstein:
3598081
EC Number:
MDL number:
UNSPSC Code:
12352209
eCl@ss:
32160406
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Assay
98%
optical activity
[α]20/D −48°, c = 1 in H2O
reaction suitability
reaction type: solution phase peptide synthesis
mp
148-151 °C (lit.)
application(s)
peptide synthesis
SMILES string
Cl[H].[H][C@]1(CCCN1)C(=O)OCc2ccccc2
InChI
1S/C12H15NO2.ClH/c14-12(11-7-4-8-13-11)15-9-10-5-2-1-3-6-10;/h1-3,5-6,11,13H,4,7-9H2;1H/t11-;/m0./s1
InChI key
NEDMOHHWRPHBAL-MERQFXBCSA-N
Looking for similar products? Visit Product Comparison Guide
Related Categories
Application
Extensive applications in peptide chemistry and used as a chiral auxiliary in the asymmetric Diels-Alder reaction.
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
A Córdova et al.
Bioorganic & medicinal chemistry letters, 9(21), 3119-3122 (1999-11-24)
Mesylates or tosylates of delta-hydroxy-L-norvaline esters spontaneously afford L-proline esters upon exposure to aqueous buffer in near quantitative yield. This novel reaction has led to the development of a simple route to optically active proline esters.
Enantiomeric purity determination of L-proline benzyl ester by chiral column gas chromatography.
M Jemal et al.
Journal of chromatography, 392, 442-446 (1987-04-17)
Aldrichimica Acta, 23, 45-45 (1990)
Yukihiro Yamamoto et al.
Applied and environmental microbiology, 76(18), 6180-6185 (2010-08-03)
We specifically examined an exopeptidase, prolyl aminopeptidase (PAP), as a target for synthesis of proline-containing peptides. A PAP from Streptomyces thermoluteus subsp. fuscus NBRC14270 (PAP14270) was obtained using sequence-based screening. From PAP14270, 144Ser was replaced by Cys (scPAP14270) to give
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service