358002
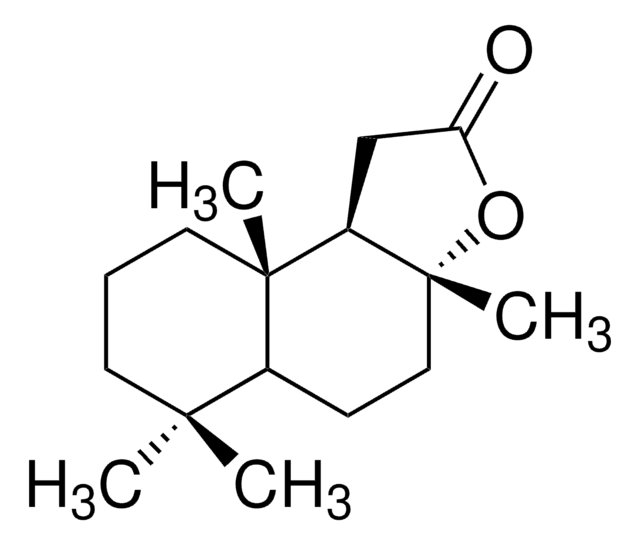
(3aR)-(+)-Sclareolide
97%
Synonym(s):
(+)-Norambreinolide
Sign Into View Organizational & Contract Pricing
All Photos(1)
About This Item
Empirical Formula (Hill Notation):
C16H26O2
CAS Number:
Molecular Weight:
250.38
EC Number:
MDL number:
UNSPSC Code:
12352005
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Quality Level
Assay
97%
form
powder
optical activity
[α]20/D +47°, c = 2% in chloroform
mp
124-126 °C (lit.)
functional group
ester
SMILES string
[H][C@@]12CC[C@@]3(C)OC(=O)C[C@]3([H])[C@@]1(C)CCCC2(C)C
InChI
1S/C16H26O2/c1-14(2)7-5-8-15(3)11(14)6-9-16(4)12(15)10-13(17)18-16/h11-12H,5-10H2,1-4H3/t11-,12+,15-,16+/m0/s1
InChI key
IMKJGXCIJJXALX-SHUKQUCYSA-N
Looking for similar products? Visit Product Comparison Guide
Related Categories
General description
Sclareolide is a diterpenoid compound mainly used in the perfume industry for its amber like odor.
Application
(3aR)-(+)-Sclareolide may be used in the total syntheses of bioactive compounds such as (+)-chloropuupehenone, (+)-chloropuupehenol, cyslabdan, acuminolide and 17-O-acetylacuminolide. It may also be used in the preparation of γ-bicyclohomofarnesal, an ambergris odorant.
Storage Class Code
11 - Combustible Solids
WGK
WGK 2
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Synthesis of Acuminolide and 17-O-Acetylacuminolide from (+)-Sclareolide.
Zoretic PA, et al.
The Journal of Organic Chemistry, 63(4), 1156-1161 (1998)
Superacid cyclization of homo-and bishomoisoprenoid acids.
Vlad PF, et al.
Chemistry of Heterocyclic Compounds, 27(3), 246-249 (1991)
Total syntheses of (+)-chloropuupehenone and (+)-chloropuupehenol and their analogues and evaluation of their bioactivities.
Hua DH, et al.
The Journal of Organic Chemistry, 69(18), 6065-6078 (2004)
Natural sesquiterpenoids.
Fraga BM.
Natural Product Reports, 20(4), 392-413 (2003)
Synthesis and Structural Revision of Cyslabdan.
Ohtawa M, et al.
Chemical & Pharmaceutical Bulletin, 64(9), 1370-1377 (2016)
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service