345350
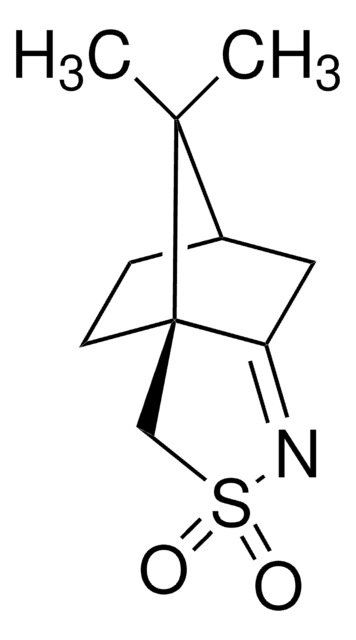
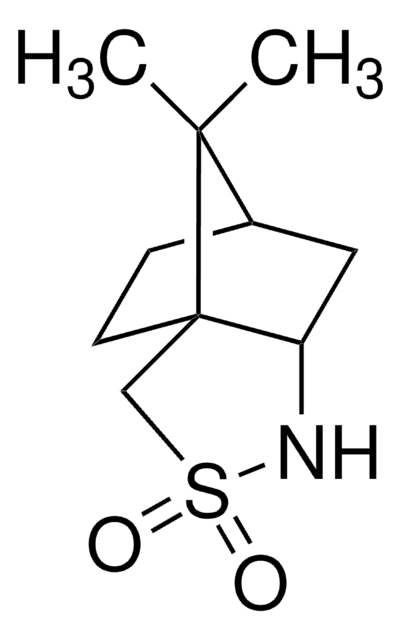
(1S)-(+)-(10-Camphorsulfonyl)oxaziridine
Synonym(s):
(1S)-(+)-(Camphorylsulfonyl)oxaziridine, (1S)-(+)-2,N-Epoxy-exo-10,2-bornanesultam
About This Item
Recommended Products
form
solid
Quality Level
optical activity
[α]28/D +45°, c = 2 in chloroform
impurities
<1% toluene
mp
172-174 °C (lit.)
storage temp.
2-8°C
SMILES string
CC1(C)[C@@H]2CC[C@]13CS(=O)(=O)N4O[C@@]34C2
InChI
1S/C10H15NO3S/c1-8(2)7-3-4-9(8)6-15(12,13)11-10(9,5-7)14-11/h7H,3-6H2,1-2H3/t7-,9+,10+,11?/m1/s1
InChI key
GBBJBUGPGFNISJ-YDQXZVTASA-N
Looking for similar products? Visit Product Comparison Guide
Related Categories
Application
- To convert prochiral ketone enolates into optically active α-hydroxy ketones via enantioselective asymmetric oxidation.
- In the synthesis of thymidine oligonucleotides connected through pyrophosphates.
- In the asymmetric synthesis of proton pump inhibitors like (R)-Rabeprazole sodium and (R)-Lansoprazole sodium from the corresponding DBU salt of prochiral sulfide.
- In the preparation of phosphonoacetate and thiophosphonoacetate oligodeoxynucleotides by oxidizing the corresponding phosphinoacetate.
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Personal Protective Equipment
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Global Trade Item Number
| SKU | GTIN |
|---|---|
| 345350-1G | 4061826760635 |
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service