All Photos(1)
About This Item
Empirical Formula (Hill Notation):
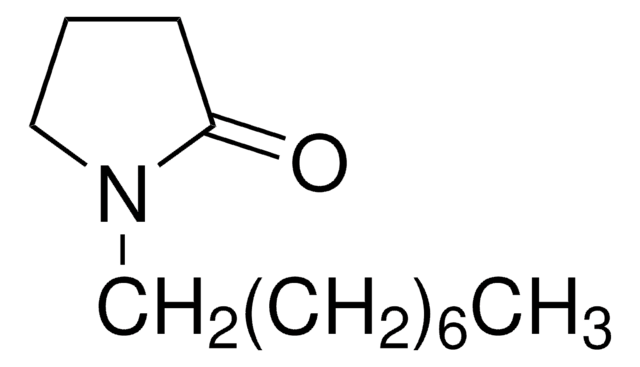
C16H31NO
CAS Number:
Molecular Weight:
253.42
EC Number:
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Quality Level
Assay
99%
form
viscous liquid
refractive index
n20/D 1.466 (lit.)
bp
202-205 °C/11 mmHg (lit.)
density
0.89 g/mL at 25 °C (lit.)
SMILES string
CCCCCCCCCCCCN1CCCC1=O
InChI
1S/C16H31NO/c1-2-3-4-5-6-7-8-9-10-11-14-17-15-12-13-16(17)18/h2-15H2,1H3
InChI key
NJPQAIBZIHNJDO-UHFFFAOYSA-N
Looking for similar products? Visit Product Comparison Guide
General description
1-Dodecyl-2-pyrrolidinone is an N-alkyl lactam and its reduction with LiH3BNMe2 to the corresponding amine has been reported. It is a potential melatonin-specific chemical penetration enhancer.
Application
1-Dodecyl-2-pyrrolidinone was used in the preparation of few-layered flakes of molybdenum disulfide (MoS2) via liquid phase exfoliation of bulk MoS2 powder.
Signal Word
Danger
Hazard Statements
Precautionary Statements
Hazard Classifications
Aquatic Acute 1 - Aquatic Chronic 1 - Skin Corr. 1B - Skin Sens. 1
Storage Class Code
8A - Combustible corrosive hazardous materials
WGK
WGK 2
Flash Point(F)
235.4 °F - closed cup
Flash Point(C)
113 °C - closed cup
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
S Sato et al.
Chemical & pharmaceutical bulletin, 46(5), 831-836 (1998-06-11)
The enhancing effects of N-dodecyl-2-pyrrolidone (NDP) on the percutaneous absorption of doxifluridine (DOX), 5-fluorouracil (5-FU), tegafur (TEG) and carmofur (CAR) were examined using an in vitro penetration technique and rat skin. Phosphate buffered isotonic saline (PBS), propylene glycol (PG) and
Andrew Winchester et al.
ACS applied materials & interfaces, 6(3), 2125-2130 (2014-01-22)
We report on the electrochemical charge storage behavior of few-layered flakes of molybdenum disulfide (MoS2) obtained by liquid phase exfoliation of bulk MoS2 powder in 1-dodecyl-2-pyrrolidinone. The specific capacitances of the exfoliated flakes obtained using a 6 M KOH aqueous
Srinivas S Godavarthy et al.
Journal of pharmaceutical sciences, 98(11), 4085-4099 (2009-08-22)
One promising way to breach the skin's natural barrier to drugs is by the application of chemicals called penetration enhancers. However, identifying potential enhancers is difficult and time consuming. We have developed a virtual screening algorithm for generating potential chemical
Vijay Krishna Rachakonda et al.
Pharmaceutical research, 25(11), 2697-2704 (2008-08-07)
A novel technique is presented for identifying potential chemical penetration enhancers (CPEs) based on changes in the electrical resistance of skin. Specifically, a multi-well resistance chamber was designed and constructed to facilitate more rapid determination of the effect of CPEs
H Sasaki et al.
The Journal of pharmacy and pharmacology, 42(3), 196-199 (1990-03-01)
The enhancing effect of combining 1-methyl-2-pyrrolidone (MP) and 1-lauryl-2-pyrrolidone (LP) as the vehicles for transdermal penetration of phenolsulphonphthalein (phenol red) has been investigated by using an in-vitro technique with excised rat skin. LP had a higher enhancing effect on the
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service