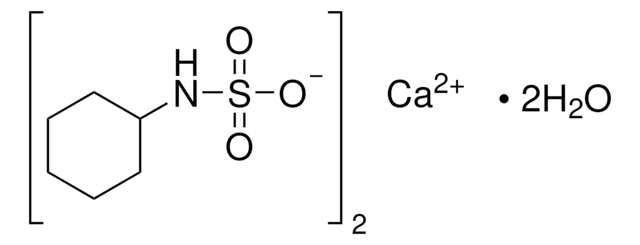29550
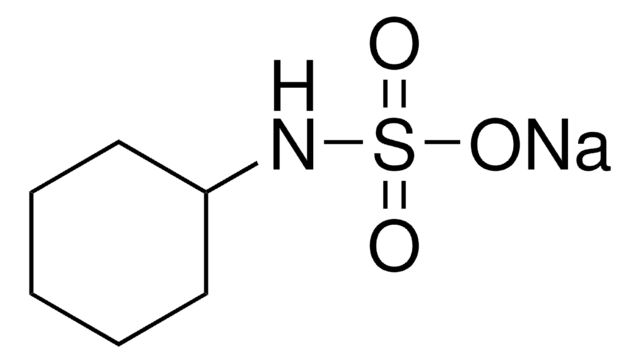
N-Cyclohexylsulfamic acid
≥98.0% (T)
Synonym(s):
Cyclamic acid, Cyclohexanesulfamic acid
Sign Into View Organizational & Contract Pricing
All Photos(1)
About This Item
Empirical Formula (Hill Notation):
C6H13NO3S
CAS Number:
Molecular Weight:
179.24
Beilstein:
2208885
EC Number:
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Assay
≥98.0% (T)
form
solid
mp
~180 °C (dec.)
solubility
dioxane: 1 g/10 mL, clear, colorless (hot)
SMILES string
OS(=O)(=O)NC1CCCCC1
InChI
1S/C6H13NO3S/c8-11(9,10)7-6-4-2-1-3-5-6/h6-7H,1-5H2,(H,8,9,10)
InChI key
HCAJEUSONLESMK-UHFFFAOYSA-N
Looking for similar products? Visit Product Comparison Guide
Related Categories
General description
The N-cyclohexylsulfamic acid salts of well−known therapeutic agents were prepared.
Storage Class Code
11 - Combustible Solids
WGK
WGK 2
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Modification of physical properties of certain antitussive and antihistaminic agents by formation of N-cyclohexylsulfamate salts.
J A CAMPBELL et al.
Journal of pharmaceutical sciences, 51, 931-934 (1962-10-01)
Lin Yi et al.
Analytical and bioanalytical chemistry, 386(3), 666-674 (2006-05-26)
Characterizing the biological effects of metabolic transformations (or biotransformation) is one of the key steps in developing safe and effective pharmaceuticals. Sulfate conjugation, one of the major phase II biotransformations, is the focus of this study. While this biotransformation typically
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service