All Photos(1)
About This Item
Empirical Formula (Hill Notation):
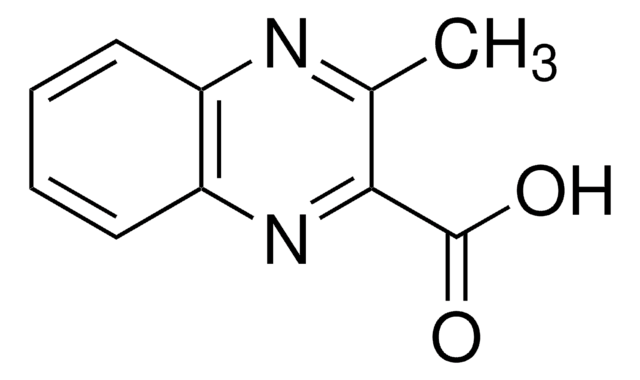
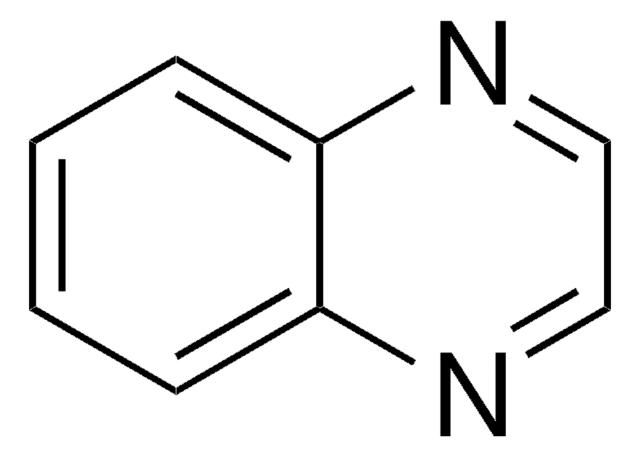
C9H6N2O2
CAS Number:
Molecular Weight:
174.16
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Assay
97%
mp
208 °C (dec.) (lit.)
functional group
carboxylic acid
SMILES string
OC(=O)c1cnc2ccccc2n1
InChI
1S/C9H6N2O2/c12-9(13)8-5-10-6-3-1-2-4-7(6)11-8/h1-5H,(H,12,13)
InChI key
UPUZGXILYFKSGE-UHFFFAOYSA-N
General description
Linear and Freundlich adsorption isotherm coefficient of 2-quinoxalinecarboxylic acid has been evaluated.
Application
2-Quinoxalinecarboxylic acid has been used in the preparation of:
- N-(2-quinoxaloyl)-α-amino acids
- bisquinoxaloyl (bisquinoxalinecarbonyl) derivatives
Signal Word
Warning
Hazard Statements
Precautionary Statements
Hazard Classifications
Eye Irrit. 2 - Skin Irrit. 2 - STOT SE 3
Target Organs
Respiratory system
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
M D Rose et al.
Food additives and contaminants, 12(2), 177-183 (1995-03-01)
A method for the determination of residues of quinoxaline-2-carboxylic acid (QCA), the major metabolite of carbadox, in pig kidney has been developed. Tissue samples were subjected to alkaline hydrolysis, liquid-liquid extractions, ion-exchange chromatography and further extraction to concentrate the analyte
Yukun Yang et al.
Biosensors & bioelectronics, 47, 475-481 (2013-04-30)
Quinoxaline-2-carboxylic acid (QCA) is difficult to measure since only trace levels are present in commercial meat products. In this study, a rapid, sensitive and selective molecularly imprinted electrochemical sensor for QCA determination was successfully constructed by combination of a novel
Yujie Wu et al.
Journal of chromatography. A, 1146(1), 1-7 (2007-03-06)
A method of high-performance liquid chromatography with UV detection has been established for simultaneous quantitative determination of quinoxaline-2-carboxylic acid (QCA) and methyl-3-quinoxaline-2-carboxylic acid (MQCA), the marker residues for carbadox (CBX) and olaquindox (OLA), respectively, in the muscles and livers of
N Prabavathi et al.
Spectrochimica acta. Part A, Molecular and biomolecular spectroscopy, 92, 325-335 (2012-03-27)
The FTIR and FT-Raman spectra of 2-quinoxaline carboxylic acid (2-QCA) has been recorded in the region 4000-450 and 4000-100 cm(-1), respectively. The conformational analysis, optimized geometry, frequency and intensity of the vibrational bands of 2-QCA were obtained by the density
Dapeng Peng et al.
Food chemistry, 237, 290-296 (2017-08-03)
An immunoaffinity column (IAC) for the selective purification of 3-methyl-quinoxaline-2-carboxylic acid (MQCA) from porcine muscle and the liver as well as the methods for its determination by high-performance liquid chromatography with ultraviolet detection (HPLC-UV) and a colloidal gold-based immunochromatographic assay
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service