290084
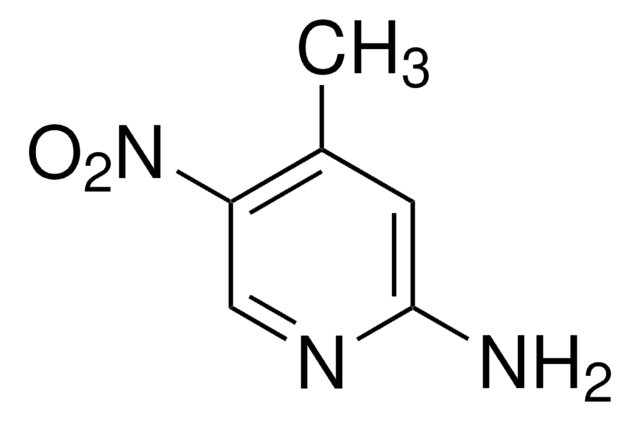
2-Amino-4-methyl-3-nitropyridine
98%
Synonym(s):
2-Amino-3-nitro-4-picoline
Sign Into View Organizational & Contract Pricing
All Photos(1)
About This Item
Empirical Formula (Hill Notation):
C6H7N3O2
CAS Number:
Molecular Weight:
153.14
Beilstein:
139111
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Assay
98%
mp
136-140 °C (lit.)
functional group
nitro
SMILES string
Cc1ccnc(N)c1[N+]([O-])=O
InChI
1S/C6H7N3O2/c1-4-2-3-8-6(7)5(4)9(10)11/h2-3H,1H3,(H2,7,8)
InChI key
IKMZGACFMXZAAT-UHFFFAOYSA-N
Looking for similar products? Visit Product Comparison Guide
General description
The crystal structure of 2-amino-4-methyl-3-nitropyridine was elucidated.
Application
2-Amino-4-methyl-3-nitropyridine was used in the synthesis of 2-amino-5-hydroxy-4-methyl-3-nitropyridine, 2-amino-4-hydroxymethyl-3-nitropyridine and 2-amino-4-methyl-3-nitropyridine-1-oxide by undergoing biotransformation by Cunninghamella elegans ATCC 26269.
Signal Word
Warning
Hazard Statements
Precautionary Statements
Hazard Classifications
Eye Irrit. 2 - Skin Irrit. 2 - STOT SE 3
Target Organs
Respiratory system
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Thomas Tully et al.
Journal of industrial microbiology & biotechnology, 39(12), 1789-1799 (2012-08-29)
Biotransformation of the highly substituted pyridine derivative 2-amino-4-methyl-3-nitropyridine by Cunninghamella elegans ATCC 26269 yielded three products each with a molecular weight of 169 Da which were identified as 2-amino-5-hydroxy-4-methyl-3-nitropyridine, 2-amino-4-hydroxymethyl-3-nitropyridine, and 2-amino-4-methyl-3-nitropyridine-1-oxide. Biotransformation by Streptomyces antibioticus ATCC 14890 gave two different
I Bryndal et al.
Spectrochimica acta. Part A, Molecular and biomolecular spectroscopy, 96, 952-962 (2012-09-04)
The crystal structures of 2-amino-4-methyl-3-nitropyridine (I), 2-amino-4-methyl-3,5-dinitropyridine (II) and 2-amino-4-methyl-5-nitropyridine (III) have been determined. The compounds crystallize in the monoclinic P2(1)/n, triclinic P-1 and monoclinic C2/c space groups, respectively. These structures are stabilized by a combination of N-H···N and N-H···O
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service