282618
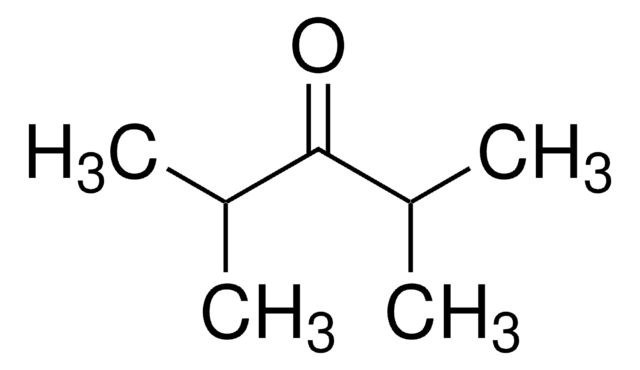
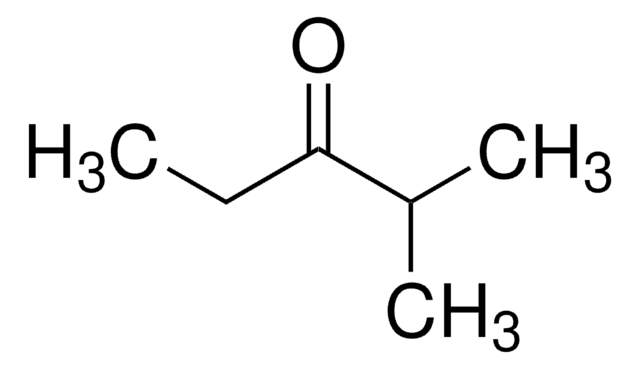
2,2,4,4-Tetramethyl-3-pentanone
98%
Synonym(s):
Di-tert-butyl ketone, Hexamethylacetone, Pivalone
Sign Into View Organizational & Contract Pricing
All Photos(1)
About This Item
Linear Formula:
(CH3)3CCOC(CH3)3
CAS Number:
Molecular Weight:
142.24
Beilstein:
1701122
EC Number:
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Assay
98%
form
liquid
refractive index
n20/D 1.419 (lit.)
bp
152-153 °C (lit.)
density
0.824 g/mL at 25 °C (lit.)
functional group
ketone
SMILES string
CC(C)(C)C(=O)C(C)(C)C
InChI
1S/C9H18O/c1-8(2,3)7(10)9(4,5)6/h1-6H3
InChI key
UIQGEWJEWJMQSL-UHFFFAOYSA-N
Looking for similar products? Visit Product Comparison Guide
General description
The Microtox EC50 values for 2,2,4,4-tetramethyl-3-pentanone has been reported. The kinetics, stoichiometry and products of the reduction reaction of 2,2,4,4-tetramethyl-3-pentanone using lithium triethylborohydride under standard conditions (tetrahydrofuran, 0°C) has been examined.
Signal Word
Warning
Hazard Statements
Precautionary Statements
Hazard Classifications
Flam. Liq. 3
Storage Class Code
3 - Flammable liquids
WGK
WGK 3
Flash Point(F)
93.2 °F - closed cup
Flash Point(C)
34 °C - closed cup
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Selective reductions. 26. Lithium triethylborohydride as an exceptionally powerful and selective reducing agent in organic synthesis. Exploration of the reactions with selected organic compounds containing representative functional groups.
Brown HC, et al.
The Journal of Organic Chemistry, 45(1), 1-12 (1980)
H F Chen et al.
Ecotoxicology and environmental safety, 30(2), 120-123 (1995-03-01)
The Microtox EC50 values for the following ketones are reported in the following homologous series: straight chain methyl ketones (acetone, 2-butanone, 2-pentanone, 2-hepatonone, 2-octanone, 2-decanone, and 2-tridecanone); methyl ketones substituted at one alpha carbon (3-methyl-2-butanone; 3,3-dimethyl-2-butanone); methyl substituted at two
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service