274186
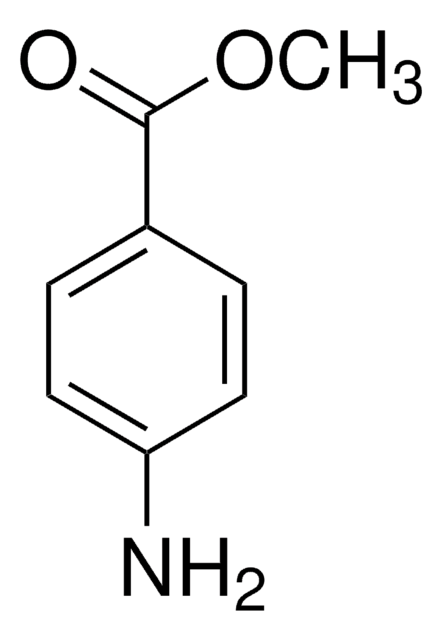
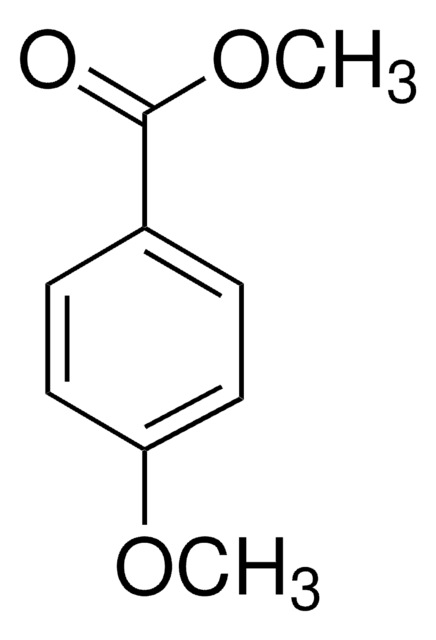
Methyl 4-aminobenzoate
98%
Synonym(s):
(4-(Methoxycarbonyl)phenyl)amine, 4-(Carbomethoxy)aniline, 4-Aminobenzenecarboxylic acid methyl ester, 4-Aminobenzoic acid methyl ester, Methyl aniline-4-carboxylate, Methyl p-aminobenzoate, p-Aminobenzoic acid methyl ester, p-Carbomethoxyaniline
About This Item
Recommended Products
Quality Level
Assay
98%
form
solid
mp
110-111 °C (lit.)
functional group
ester
SMILES string
COC(=O)c1ccc(N)cc1
InChI
1S/C8H9NO2/c1-11-8(10)6-2-4-7(9)5-3-6/h2-5H,9H2,1H3
InChI key
LZXXNPOYQCLXRS-UHFFFAOYSA-N
Looking for similar products? Visit Product Comparison Guide
Related Categories
General description
Application
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Personal Protective Equipment
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Global Trade Item Number
| SKU | GTIN |
|---|---|
| 274186-25G | 4061826151877 |
| 274186-100G | 4061838348340 |
| 274186-5G | 4061826151884 |
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service