All Photos(1)
About This Item
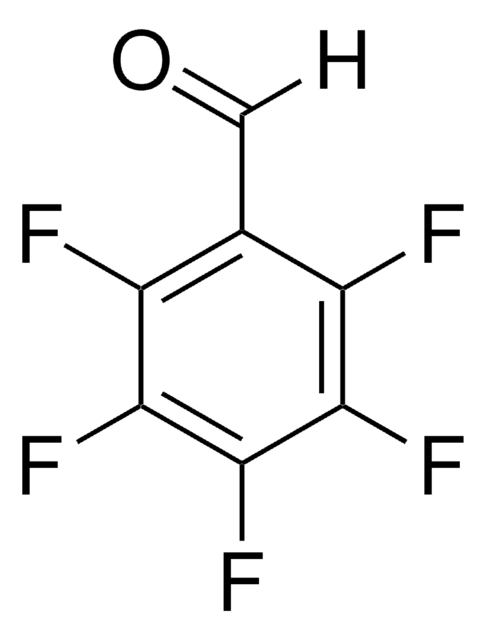
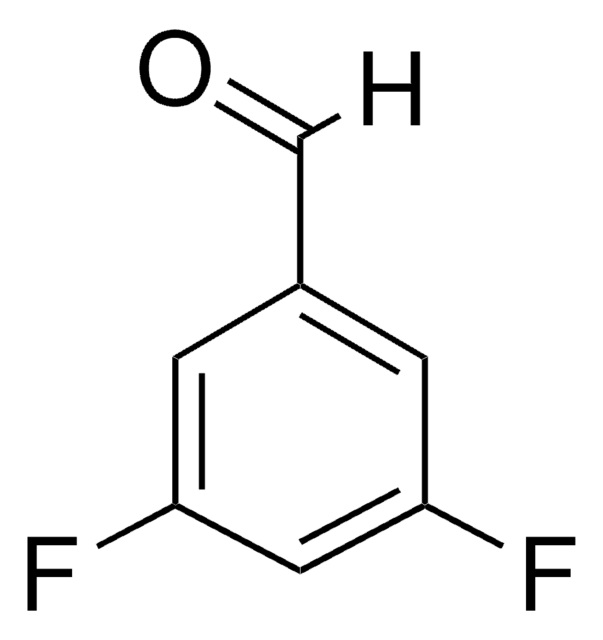
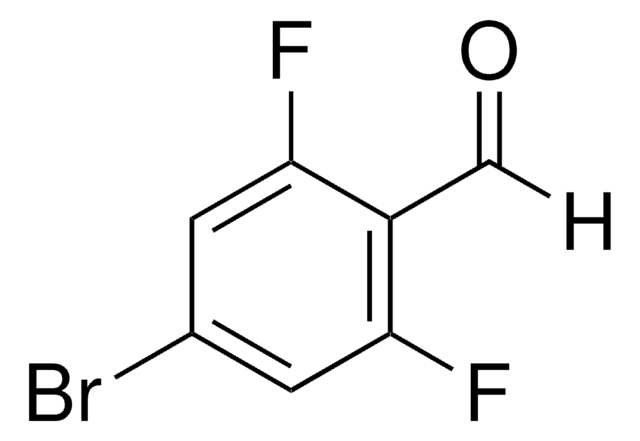
Linear Formula:
F2C6H3CHO
CAS Number:
Molecular Weight:
142.10
Beilstein:
1935273
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Assay
98%
form
liquid
refractive index
n20/D 1.502 (lit.)
bp
82-84 °C/15 mmHg (lit.)
mp
15-17 °C (lit.)
density
1.317 g/mL at 25 °C (lit.)
functional group
aldehyde
storage temp.
2-8°C
SMILES string
Fc1cccc(F)c1C=O
InChI
1S/C7H4F2O/c8-6-2-1-3-7(9)5(6)4-10/h1-4H
InChI key
SOWRUJSGHKNOKN-UHFFFAOYSA-N
Looking for similar products? Visit Product Comparison Guide
Application
2,6-Difluorobenzaldehyde can be used as a reactant to synthesize:
- 5-Cyano-6-(2,6-difluorophenyl)-5,6-dihydro-2-thiouracil via one-pot cyclocondensation reaction with ethyl cyanoacetate and thiourea.
- 1-(2,6-Difluorobenzyl)-2-(2,6-difluorophenyl)-benzimidazole by reacting with 1,2-phenylenediamine in the presence of a catalytic amount of p-toluenesulfonic acid.
- (3E)-4-(2,6-Difluorophenyl)-3-buten-2-one by Wittig olefination reaction with acetylmethylidenetriphenyl phosphorane.
- Methyl 4-fluorobenzo[b]thiophene-2-carboxylate by treating with methyl thioglycolate in the presence of K2CO3.
Signal Word
Danger
Hazard Statements
Precautionary Statements
Hazard Classifications
Aquatic Chronic 3 - Eye Dam. 1 - Skin Corr. 1C
Storage Class Code
8A - Combustible corrosive hazardous materials
WGK
WGK 2
Flash Point(F)
180.5 °F - closed cup
Flash Point(C)
82.5 °C - closed cup
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
4-Substituted (benzo [b] thiophene-2-carbonyl) guanidines as novel Na+/H+ exchanger isoform-1 (NHE-1) inhibitors
Lee Sunkyung, et al.
Bioorganic & Medicinal Chemistry Letters, 15(12), 2998-3001 (2005)
C Cabrera et al.
AIDS research and human retroviruses, 15(17), 1535-1543 (1999-12-02)
Negatively charged albumins (NCAs) have been identified as potent inhibitors of HIV-1 replication in vitro. Time of addition studies suggest that succinylated and aconitylated human serum albumin (Suc-HSA and Aco-HSA) act at an early stage of the virus life cycle
A systematic method of promoting an aryl fluoride to coordinate to ruthenium (11).
Inorgorganica Chimica Acta, 228(2), 127-131 (1995)
Some observations on the base-catalyzed cyclocondensation of 2, 6-dihalobenzaldehydes, ethyl cyanoacetate, and thiourea
Al-Omar Mohamed A, et al.
Synthetic Communications, 40(10), 1530-1538 (2010)
G M Wahl et al.
Proceedings of the National Academy of Sciences of the United States of America, 76(8), 3683-3687 (1979-08-01)
We describe a technique for transferring electrophoretically separated bands of double-stranded DNA from agarose gels to diazobenzyloxymethyl-paper. Controlled cleavage of the DNA in situ by sequential treatment with dilute acid, which causes partial depurination, and dilute alkali, which causes cleavage
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service