All Photos(1)
About This Item
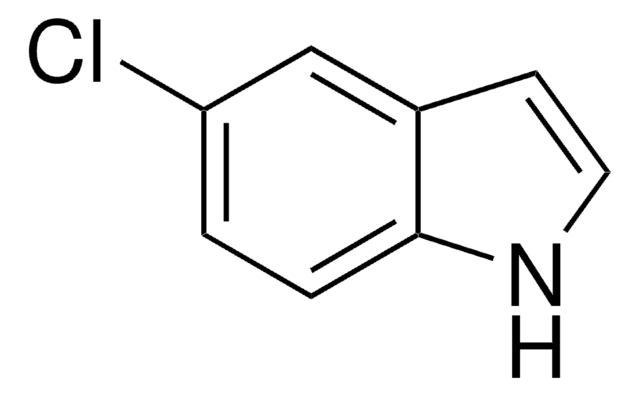
Empirical Formula (Hill Notation):
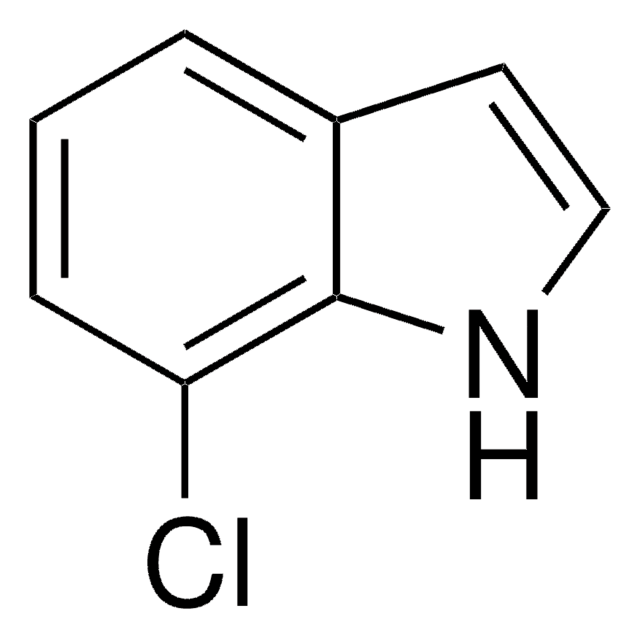
C8H6ClN
CAS Number:
Molecular Weight:
151.59
Beilstein:
114880
EC Number:
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Assay
98%
refractive index
n20/D 1.628 (lit.)
bp
129-130 °C/4 mmHg (lit.)
solubility
ethanol: 50 mg/mL, clear, colorless
density
1.259 g/mL at 25 °C (lit.)
SMILES string
Clc1cccc2[nH]ccc12
InChI
1S/C8H6ClN/c9-7-2-1-3-8-6(7)4-5-10-8/h1-5,10H
InChI key
SVLZRCRXNHITBY-UHFFFAOYSA-N
Looking for similar products? Visit Product Comparison Guide
General description
The nitrosation rate of 4-chloroindole and the stability of its nitrosated products were studied.
Signal Word
Warning
Hazard Statements
Precautionary Statements
Hazard Classifications
Eye Irrit. 2 - Skin Irrit. 2 - STOT SE 3
Target Organs
Respiratory system
Storage Class Code
10 - Combustible liquids
WGK
WGK 3
Flash Point(F)
235.4 °F - closed cup
Flash Point(C)
113 °C - closed cup
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Zhi-Gang Zhang et al.
Journal of biotechnology, 139(1), 12-18 (2008-11-06)
Cytochrome P450 (P450) 2A6 is able to catalyze indole hydroxylation to form the blue dye indigo. The wild-type P450 2A6 enzyme was randomly mutated throughout the whole open reading frame and screened using 4-chloroindole hydroxylation, a substituted indole selected from
H G Tiedink et al.
Cell biology and toxicology, 7(4), 371-386 (1991-10-01)
4-chloro-methoxyindole is a naturally occurring compound in Vicia faba which can easily react with nitrite to form a N-nitroso compound. In this in vitro study, the potential genotoxic effects of nitrosated 4-chloro-6-methoxyindole and its structural analogue 4-chloroindole were evaluated for
Zhong-Liu Wu et al.
The Journal of biological chemistry, 280(49), 41090-41100 (2005-10-11)
The natural product indole is a substrate for cytochrome P450 2A6. Mutagenesis of P450 2A6 was done to expand its capability in the oxidization of bulky substituted indole compounds, which are not substrates for the wild-type enzyme or the double
H G Tiedink et al.
Food and chemical toxicology : an international journal published for the British Industrial Biological Research Association, 27(11), 723-730 (1989-11-01)
The nitrosation rates of indole-3-acetonitrile, indole-3-carbinol, indole and 4-chloroindole and the stability of their nitrosated products were investigated. Each of the nitrosated indole compounds was directly mutagenic to Salmonella typhimurium TA100 in the following order of potency: 4-chloroindole much greater
Chaitany Jayprakash Raorane et al.
Biomolecules, 10(8) (2020-08-23)
Multi-drug resistant Acinetobacter baumannii is well-known for its rapid acclimatization in hospital environments. The ability of the bacterium to endure desiccation and starvation on dry surfaces for up to a month results in outbreaks of health care-associated infections. Previously, indole
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service