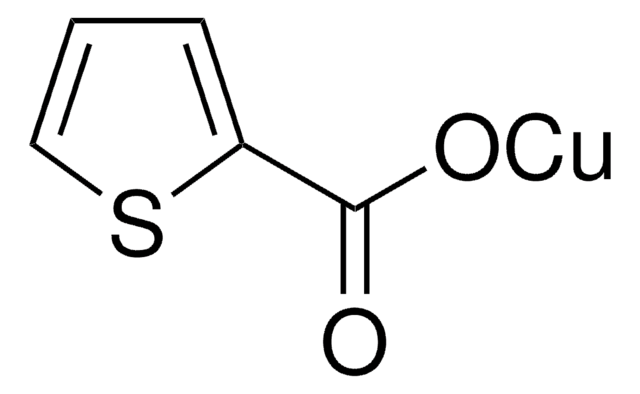
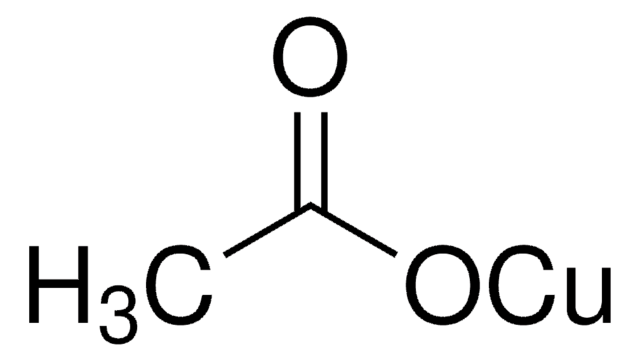
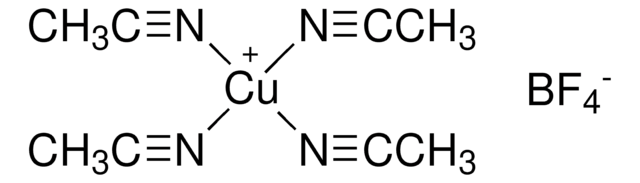
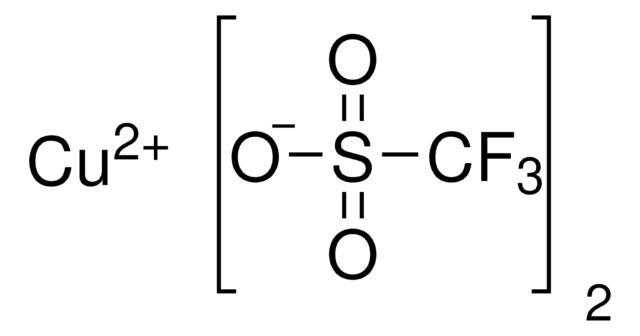230502
Copper(I) bromide dimethyl sulfide complex
99%
Synonym(s):
Copper(I) bromide methyl sulfide complex, Cuprous bromide dimethyl sulfide complex, Cuprous bromide methyl sulfide complex
Sign Into View Organizational & Contract Pricing
All Photos(2)
About This Item
Linear Formula:
CuBr · CH3SCH3
CAS Number:
Molecular Weight:
205.58
Beilstein:
4386581
EC Number:
MDL number:
UNSPSC Code:
12161600
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Quality Level
Assay
99%
form
solid
reaction suitability
core: copper
reagent type: catalyst
mp
132 °C (dec.) (lit.)
SMILES string
[Cu]Br.CSC
InChI
1S/C2H6S.BrH.Cu/c1-3-2;;/h1-2H3;1H;/q;;+1/p-1
InChI key
PMHQVHHXPFUNSP-UHFFFAOYSA-M
Looking for similar products? Visit Product Comparison Guide
Application
Catalyst employed in the addition of Grignard reagents (alkyl, alkenyl, aryl) to fullerenes. This process adds 5 organic groups to [60]fullerene and 3 groups to [70]fullerene.
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Organic Syntheses, 83, 80-80 (2006)
Beatriz Pacheco Berciano et al.
Organic letters, 12(18), 4038-4041 (2010-08-26)
The copper-catalyzed direct alkynylation of azoles with 1,1-dibromo-1-alkenes as electrophiles is described. These easily accessible substrates are a useful addition to the field of direct alkynylations in an efficient and functional group tolerant reaction to provide a straightforward entry to
Enantioselective Cu-catalyzed 1,4-addition of Grignard reagents to cyclohexenone using taddol-derived phosphine-phosphite ligands and 2-methyl-THF as a solvent.
Tobias Robert et al.
Angewandte Chemie (International ed. in English), 47(40), 7718-7721 (2008-09-06)
Rossiter, B. E. et al.B. E.; Swingle, N. M.
Chemical Reviews, 92, 771-771 (1992)
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service